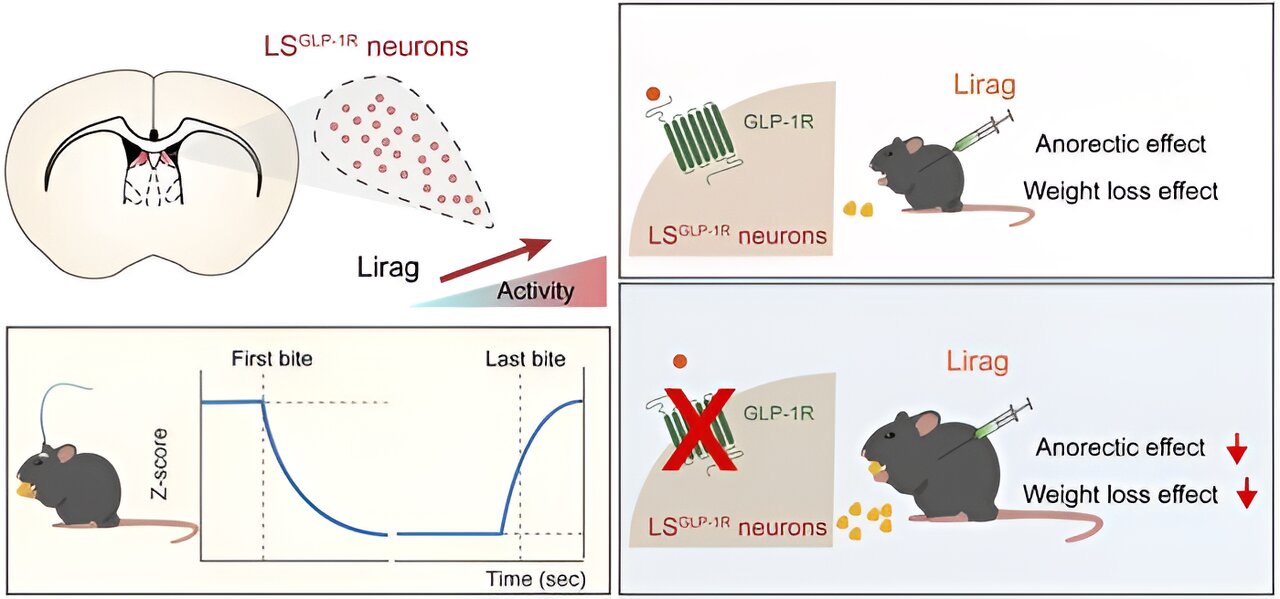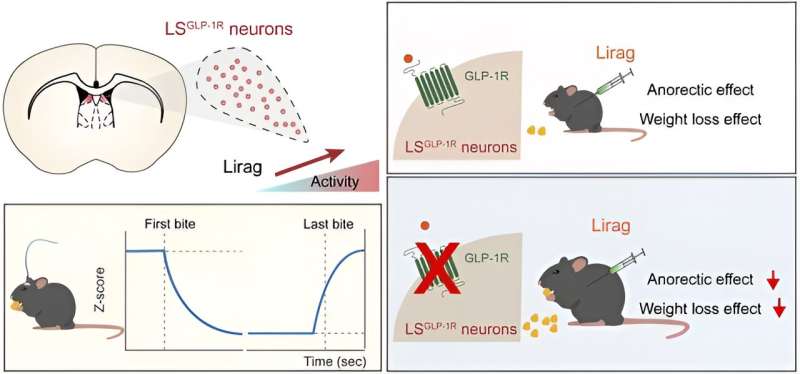
A research group led by Prof. Zhu Yingjie from the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences has revealed the essential role of lateral septum (LS) neurons in mediating anorectic and weight-lowering effects of the anti-obesity drug, liraglutide, in mice. The study was published in Journal of Clinical Investigation.
Anti-obesity medications have shown greater efficacy than lifestyle changes and diet, and have lower risks and fewer side effects than surgery. Since 2014, glucagon-like peptide-1 receptor (GLP-1R) agonists have emerged as a class of medications, significantly outperforming other weight-loss drugs in both effectiveness and safety.
GLP-1 is an incretin hormone encoded by the proglucagon gene (GCG). Its effects are mediated through GLP-1R. Liraglutide, a short-acting GLP-1R agonist, reduces appetite and slows gastric emptying, making it the first GLP-1-based anti-obesity drug on the market. Despite the widespread expression of GLP-1R in the brain, the precise neural mechanisms through which its agonists regulate food intake and body weight remain poorly understood.
In this study, researchers discovered that GLP-1Rs are abundantly expressed in the dorsal LS, and that liraglutide strongly activated GLP-1R-positive (LSGLP-1R) neurons in this region.
Knockdown of GLP-1Rs in the LS attenuated liraglutide’s effects on feeding suppression and weight-lowering, whereas targeted knockdown in the hypothalamic regions, such as the paraventricular nucleus of the hypothalamus (PVN) and arcuate nucleus of the hypothalamus (Arc), failed to replicate the effect. This suggests that GLP-1Rs in LS mediated the anorectic effect of liraglutide.
Furthermore, researchers investigated the intrinsic activity of LSGLP-1R neurons during natural feeding by using fiber photometry in freely moving mice. They observed a significant decrease in Ca2+ signals at the start of food consumption, which continued throughout the eating period and returned to baseline after feeding ended.
The activation of these neurons suppressed feeding and reduced body weight, mimicking the effects of liraglutide. Conversely, the inactivation of these neurons substantially attenuated liraglutide‘s anorectic and weight-reducing efficacy.
This study provides valuable insights into the neural mechanisms underlying feeding behavior, and paves the way for new strategies to treat eating disorders and obesity, as well as further exploration of the GLP-1 signaling pathway.
More information:
Zijun Chen et al, GLP-1R–positive neurons in the lateral septum mediate the anorectic and weight-lowering effects of liraglutide in mice, Journal of Clinical Investigation (2024). DOI: 10.1172/JCI178239
Citation:
Mouse study reveals new central action target of weight loss drug GLP-1R agonists (2024, September 5)
retrieved 6 September 2024
from https://medicalxpress.com/news/2024-09-mouse-reveals-central-action-weight.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.