In the fight against fatty liver disease, researchers are looking for any and all possible solutions. But to combat the disease, which is also known as metabolic dysfunction-associated steatotic liver disease, or MASLD, scientists must first understand how the liver metabolizes fat. MASLD is on the rise in the U.S., now affecting nearly 40% of adults. The condition occurs when the body deposits extra fat in the liver, leading to inflammation, fibrosis and, in some cases, permanent liver damage or liver cancer.
A new study by researchers at Yale School of Medicine (YSM), published Aug. 27 in the journal Cell Metabolism, finds that people with MASLD burn fat in their livers at the same rate as people without the disease. The study, whose senior author is Gerald Shulman, MD, Ph.D., George R. Cowgill Professor of Medicine (Endocrinology) and professor of cellular and molecular physiology at YSM, appears to settle this question, which has been in dispute in the field.
The researchers also found that increasing blood levels of the glucose-related hormone glucagon stimulates liver metabolism in both healthy people and people with fatty liver disease. That’s important because some experimental weight loss and diabetes drugs such as retatrutide also contain a glucagon agonist component—a welcome finding that suggests these drugs seem to be combating fatty liver disease through multiple mechanisms.
Shulman points out that, while some medications exist to treat fatty liver disease, including the newly FDA-approved drug resmetirom, these treatments don’t work for all patients.
“It’s clear that we need other agents,” he said, suggesting that therapies that rev up the liver’s mitochondrial fat oxidation could be a useful approach.
How to boost liver metabolism
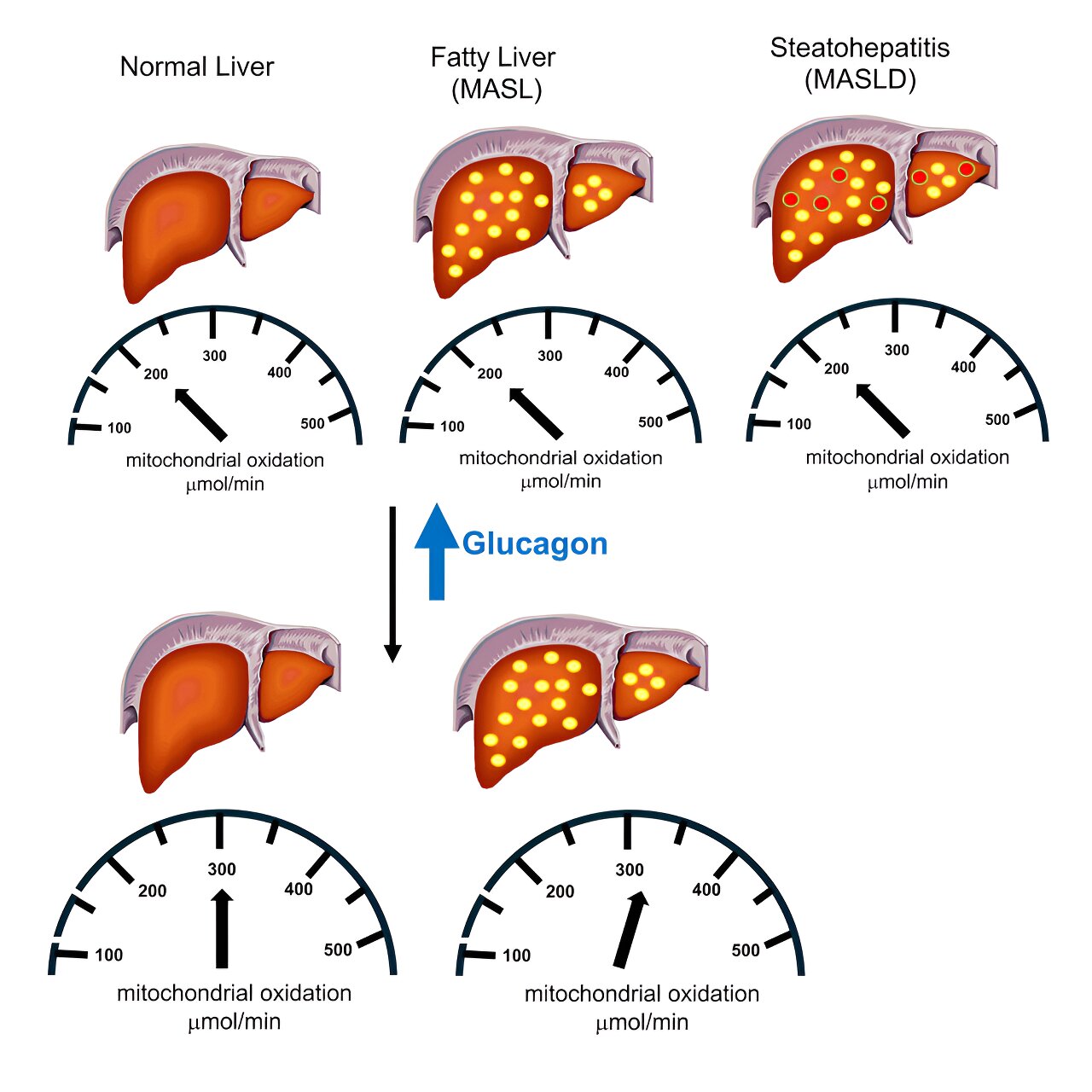
Mitochondrial oxidation is the metabolic process liver cells use to convert fat to energy. If researchers could identify a way to increase liver mitochondria’s fat-burning powers, that could reduce fat deposits in the liver and possibly even reverse MASLD and metabolic dysfunction-associated steatohepatitis (MASH).
What was unclear, however, is whether these conditions on their own increase mitochondrial oxidation, since previous studies had differing conclusions. If fatty liver already raises the organ’s fat metabolism, it seemed possible that trying to boost the metabolism even more would not have any effect against the disease.
Hoping to put that question to rest, Shulman and his team used a technique they recently developed known as positional isotopomer NMR tracer analysis, or PINTA. Many past studies have examined liver metabolism in cells or in test tubes in the lab, because it is very difficult to follow metabolic reactions as they happen in the body. But that natural context is critical and can change metabolism significantly as compared to artificial settings, Shulman said.
In their method, study volunteers were infused with a mixture of three stable (non-radioactive) isotopes that allowed the scientists to trace the metabolism of the labeled substrates (surfaces acted upon by enzymes) and quantify rates of liver mitochondrial oxidation and gluconeogenesis as it happens in the body.
Using this approach, they looked at liver fat metabolism in 12 healthy volunteers and compared it to that of 13 study volunteers with MASLD (fatty liver with inflammation) and 13 volunteers with the related condition metabolic dysfunction-associated steatotic liver, or MASL, a less severe form of fatty liver.
They found that all three groups had the same rates of mitochondrial oxidation in the liver, which contrasted with the findings of some previous studies, affirming others. They also gave study volunteers infusions of glucagon, a hormone that stimulates glucose production. The hormone bumped up rates of hepatic mitochondrial fat oxidation in the healthy volunteer and MASL groups by 50 to 75%.
“This suggests that increasing glucagon will have additive beneficial effects to reduce liver fat, not only by reducing energy intake, but also increasing hepatic energy expenditure,” Shulman said. People taking certain weight loss medications that also alleviate fatty liver have diminished appetites and generally consume fewer calories, reducing their overall energy intake.
The glucagon finding indicates that medications that boost levels of the hormone could treat fatty liver by increasing energy expenditure. It’s known that GLP-1 agonist drugs such as semaglutide (Ozempic) can alleviate excess liver fat present in MASLD by promoting weight loss and reducing overall energy intake.
Shulman and his colleagues are trying to find approaches that also increase fat metabolism. Indeed, experimental combination drugs that pair GLP-1 agonists with compounds that raise levels of glucagon have recently been found to effectively treat fatty liver disease.
Next, Shulman and his team want to use their method to test the effects of these and other metabolism-related drugs on liver metabolism. They also want to follow study volunteers with MASLD and MASH for a longer period to see whether the beneficial effects of glucagon on liver metabolism persist, which would be important for any successful treatment.
More information:
Kitt Falk Petersen et al, Glucagon promotes increased hepatic mitochondrial oxidation and pyruvate carboxylase flux in humans with fatty liver disease, Cell Metabolism (2024). DOI: 10.1016/j.cmet.2024.07.023
Citation:
Weight loss drugs could help fight fatty liver disease (2024, September 18)
retrieved 21 September 2024
from https://medicalxpress.com/news/2024-09-weight-loss-drugs-fatty-liver.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.