Researchers at Karolinska Institutet and AstraZeneca have discovered that gene expression in adipose stem cells varies according to sex and type of adipose tissue in mice.
The study, published in Nature Communications, shows that female adipose stem cells express higher levels of estrogen and progesterone receptors, while male cells express a gene that encodes for an enzyme that deactivates estrogens.
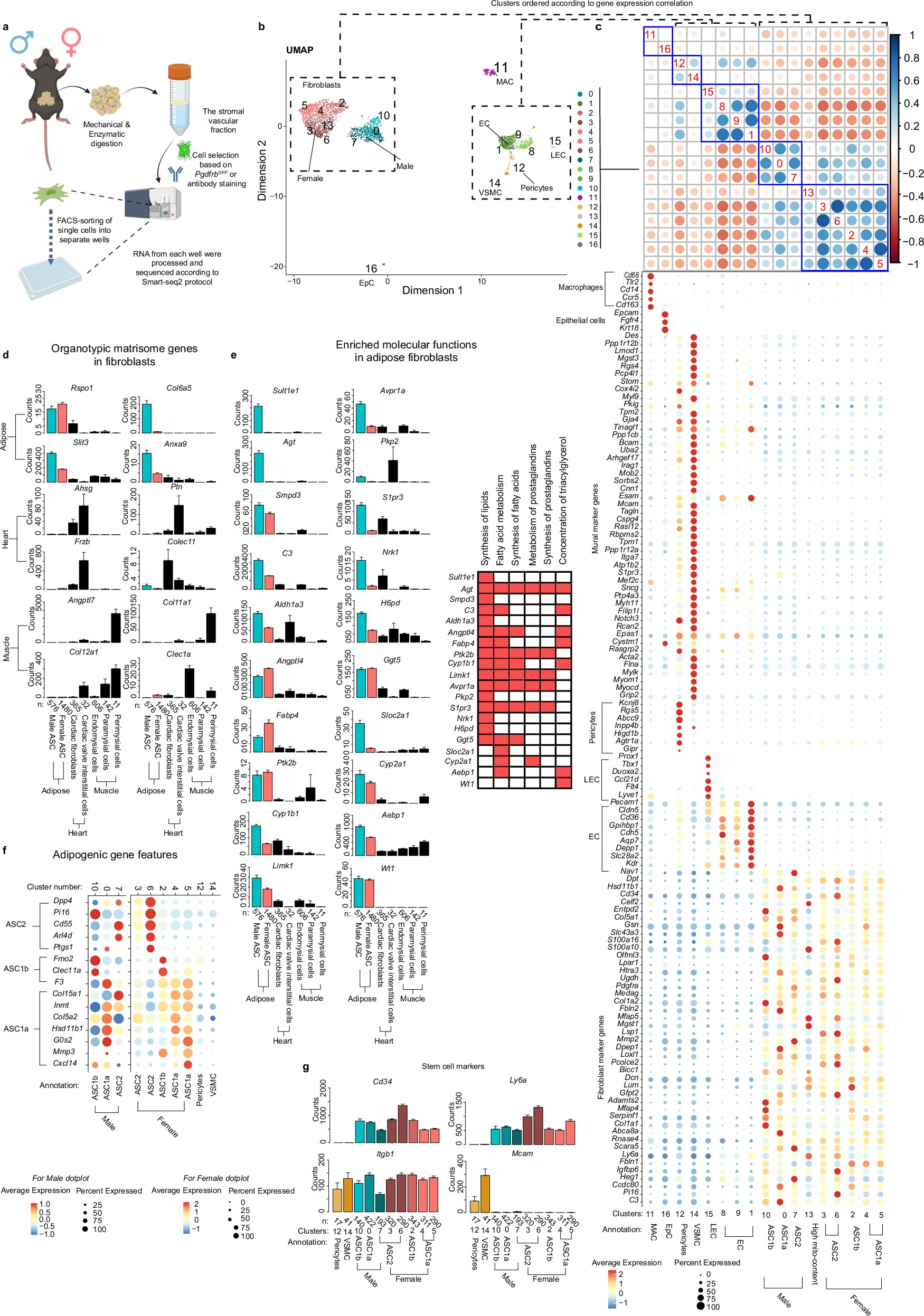
According to Martin Uhrbom, Phd Student, at the Department of Medicine, Huddinge, the team “used single cell RNA sequencing and 3D rendering imaging to better characterize adipose stem cells in mice. In addition, we have validated our findings using publicly available datasets.”
These findings may pave the way for future therapeutic interventions to increase the body’s fat storage capacity and improve metabolism.
“We have found that the gene expression of adipose stem cells in male and female mice varies depending on sex and type of adipose tissue. We present a sexually dimorphic gene signature of adipose stem cells that can guide future studies in the field,” says Uhrbom.
“For example, female adipose stem cells express higher levels of the estrogen (Esr1) and progesterone (Pgr) receptors, whereas male cells express Sulfotransferase family 1E member 1 (Sult1e1), an enzyme that deactivates estrogens. Moreover, two genes associated with higher blood pressure, angiotensin (Agt) and Angiotensin converting enzyme (Ace), are enriched in male adipose stem cells.”
Commenting on the relevance of the study, Uhrbom says, “Adipose stem cells play a crucial role in the expansion of adipose tissue, since these cells can differentiate into new mature adipocytes, that are the fat-storing units of our body.
“Over-feeding can lead to obesity and impaired metabolism of adipose tissue, which consequently can provoke unwanted redistribution of lipids to non-adipose tissue such as skeletal muscle and heart, causing insulin resistance and increased blood glucose levels.
“It is therefore highly desirable to gain more knowledge on how the fat storage capacity of adipose tissue can be increased. In this aspect, adipose stem cells may play an important role, with future therapeutic interventions aimed to increase their ability to differentiate to mature adipocytes and by doing so, increase our fat-storing capacity.
“The next step is to focus on specific signaling pathways that can influence the ability of adipose stem cells to differentiate into mature adipocytes, as well as investigating their relevance to human biology.”
More information:
Martin Uhrbom et al, Adipose stem cells are sexually dimorphic cells with dual roles as preadipocytes and resident fibroblasts, Nature Communications (2024). DOI: 10.1038/s41467-024-51867-9
Citation:
Study reveals sex-specific gene expression in adipose stem cells of mice (2024, September 17)
retrieved 21 September 2024
from https://medicalxpress.com/news/2024-09-reveals-sex-specific-gene-adipose.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.