Scientists have revealed how a population of insulin-producing cells (IPCs) in the fruit fly brain works together to produce a concerted response to metabolic demands and internal state changes.
The study, published today as a Reviewed Preprint in eLife, is described by the editors as a fundamental study in which the authors convincingly demonstrate how the diversity of responses across individual IPCs occur simultaneously and together control insulin release to maintain metabolic processes. This work will be of interest to neuroscientists and physiologists, particularly as it shows how cellular diversity results in a better control of homeostasis in short time scales.
“Insulin plays a crucial role in balancing the metabolic demands of the body. As these demands are highly dynamic, insulin release needs to be constantly adjusted,” explains co-lead author Martina Held, postdoctoral research scientist in the Ache Lab at Julius-Maximilians-University of Würzburg, Germany.
“IPCs are the main source of insulin in the fruit fly and work in the same way as IPCs in the human pancreas. Studying them can help us understand the dynamics of insulin signaling in diseases such as diabetes and metabolic syndrome.”
In a previous study, the authors showed that the nutritional state of fruit flies strongly affects the activity of IPCs. These cells were less active in starved flies compared with fed flies and became activated when the flies were fed with glucose. In the current study, they set out to understand how this small group of 14–18 IPCs in the fruit fly brain can orchestrate such complex and dynamic responses, while remaining responsive to other inputs.
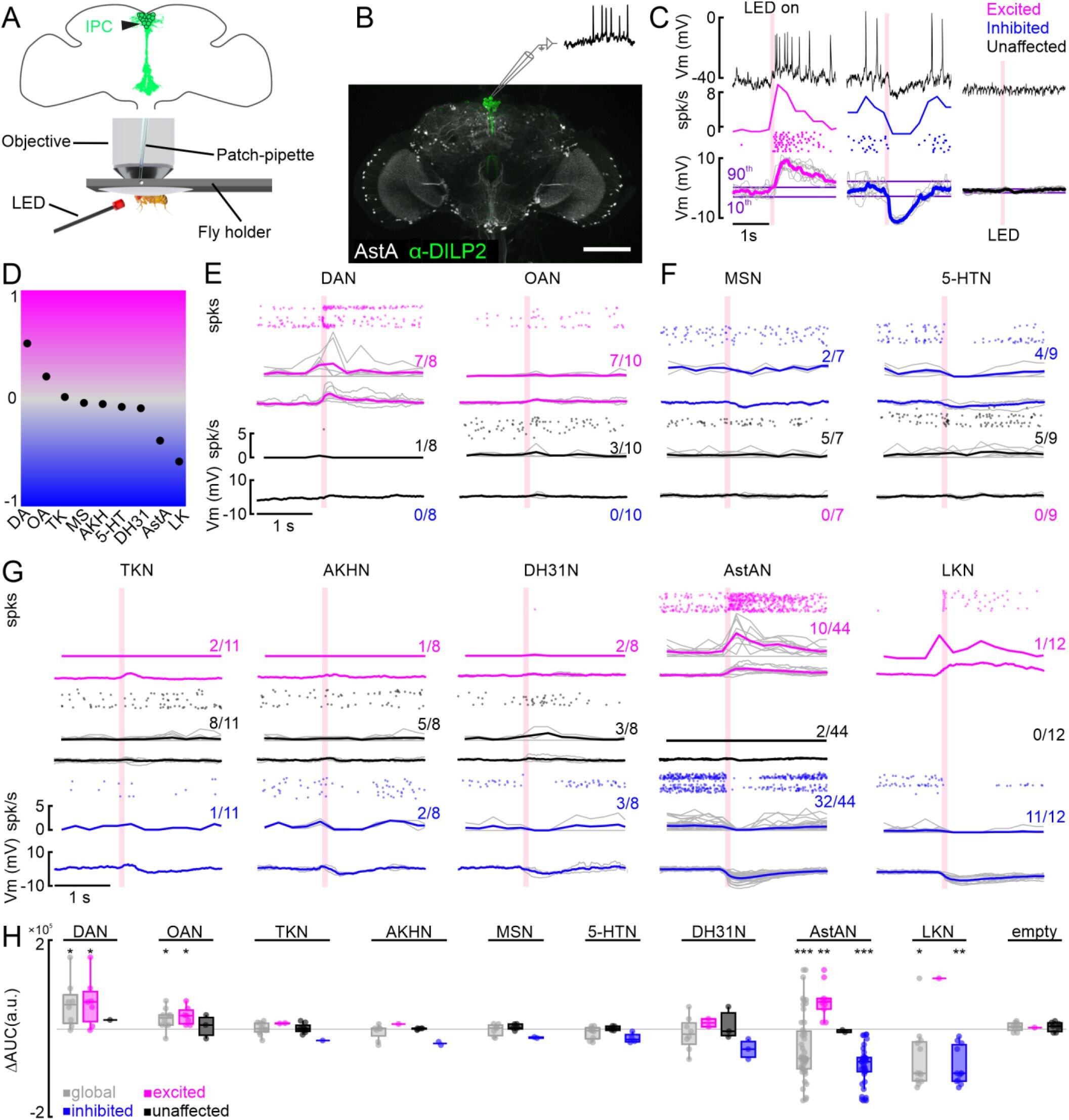
The team started by identifying the brain signal transmitters (neuromodulators) that affect IPC function, by analyzing the levels of different brain signal receivers (receptors) found in the IPCs. This revealed around 40 receptors, some of which were new, while others were already known.
The most common receptor was the insulin receptor itself, but receptors for a wide range of other molecule types including amines, neuropeptides and fast-acting neurotransmitters were also identified, showing that IPCs are intricately regulated by a wide variety of signaling molecules in the brain.
Because recent studies suggest there are functional subpopulations of IPCs, the team used gene analysis to categorize the IPCs further. This showed that neuromodulator receptors were present at different amounts in different subsets of cells.
To explore how these different neuromodulators affect IPC function, the team recorded the activity of individual IPCs while altering the neuromodulator signals they received, classifying the response of each IPC as excited, inhibited or unaffected.
To their surprise, they found that while some neuromodulators did not seem to shift activity of the overall population of cells dramatically, the same neuromodulators had different and even opposite effects on individual IPCs, with individual IPCs exhibiting all three response types (excited, inhibited and unaffected). They also found that certain neuromodulators can shift the overall population of IPCs towards an excited state, while others can shift the entire population towards an inhibited state.
Moreover, when quantifying inputs to IPCs provided by classical chemical synapses, using a connectome of the fruit fly brain, they found that although all IPCs receive signals from neurons expressing the main neurotransmitters (glutamate and acetylcholine), the strength of the individual synaptic connections differs between IPCs. This further contributes to a variable and flexible response of the IPCs to a range of dynamic inputs.
Despite this inherent variability across IPCs, the authors say the finding that the entire IPC population was shifted towards excited or inhibited state is important in light of earlier findings showing the cells’ adaptive response to glucose levels and behavioral state changes. Their results suggest that the IPCs are a fine-tunable population of cells that could either increase the responsiveness of the entire system to rapidly increase insulin release, or shift it towards an inhibited state that is less sensitive to small changes in a low-insulin demand state.
“Our study shows that the activity of insulin-producing cells in the fruit fly brain is affected by multiple neuromodulatory inputs, allowing the system to flexibly respond to rapidly changing metabolic demands and sensory inputs,” concludes senior author Jan Ache, Emmy-Noether Group Leader at Julius-Maximilians-University of Würzburg.
“The diversity in signaling systems adds flexibility and stability and, in the case of the insulin-producing cells, could explain how a relatively small population of cells integrate a large variety of signals to achieve flexible insulin release tailored to the animal’s ever-changing demands.”
More information:
Martina Held et al, Aminergic and peptidergic modulation of Insulin-Producing Cells in Drosophila, eLife (2024). DOI: 10.7554/eLife.99548.1
Citation:
Study reveals how insulin-producing cells work together to respond to metabolic demands (2024, September 10)
retrieved 13 September 2024
from https://medicalxpress.com/news/2024-09-reveals-insulin-cells-metabolic-demands.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.