Periodontal disease, which affects the gums and tissues that surround the teeth, is one of the most prevalent dental conditions worldwide. Most often caused by the formation and accumulation of bacterial biofilm around the teeth, periodontal disease can ultimately lead to tooth loss if left unattended.
Interestingly, the inflammatory effects of periodontal bacteria can go well beyond the mouth, leading to systemic effects. Over the past few decades, clinical studies have revealed that the periodontal pathogen Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) is closely related to the onset and worsening of rheumatoid arthritis (RA), a serious autoimmune disease that affects joints. However, what goes down at the molecular level remains largely unexplored and unclear.
In a recent study published in the International Journal of Oral Science, a research team from Tokyo Medical and Dental University (TMDU) in Japan sought to fill this knowledge gap through detailed mechanistic studies in an animal model.
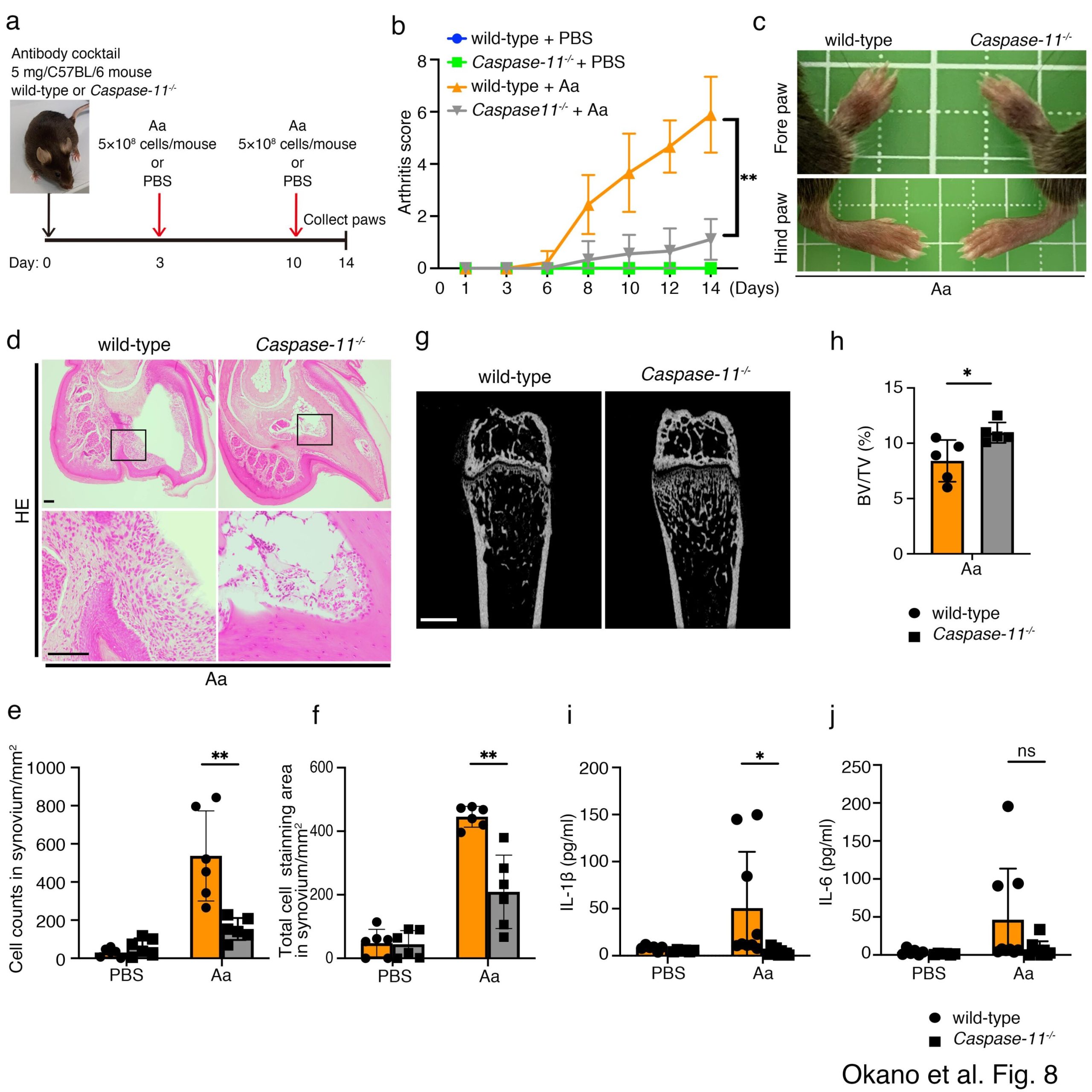
First, the researchers conducted preliminary experiments to confirm whether A. actinomycetemcomitans infection influenced arthritis in mice. To this end, they used the collagen antibody-induced arthritis mouse model, which is a well-established experimental model that mimics several aspects of RA in humans.
They found that infection with this specific bacterium led to increased limb swelling, cellular infiltration into the lining of the joints, and higher levels of the inflammatory cytokine interleukin-1β (IL-1β) within the limbs.
Notably, these symptoms of worsening RA could be suppressed by administering a chemical agent called clodronate that depletes macrophages—a type of immune cell. This demonstrated that macrophages were somehow involved in aggravating RA caused by A. actinomycetemcomitans infection.
Further investigation using macrophages derived from mouse bone marrow revealed that A. actinomycetemcomitans infection increased the production of IL-1β. In turn, this triggered the activation of a multiprotein complex known as the inflammasome, which plays a key role in initiating and modulating the body’s inflammatory response to infections.
The researchers added yet one more piece to this puzzle using caspase-11-deficient mice. In these animals, inflammasome activation due to A. actinomycetemcomitans was suppressed. Most importantly, caspase-11-deficient mice exhibited less deterioration of arthritis symptoms, hinting at the important role that caspase-11 plays in this context.
“Our research findings provide new insights into the link between periodontal pathogenic bacteria and the exacerbation of arthritis through inflammasome activation, offering important information on the long-debated relationship between periodontal disease and systemic diseases,” says Professor Toshihiko Suzuki, one of the senior authors of the study.
“The findings of this research may pave the way for advances in clinical treatments for RA induced by infection with A. actinomycetemcomitans. Our suggestion to inhibit inflammasome activation could attenuate the expansion of inflammation to joints, resulting in a recovery from arthritis symptoms,” says lead author Dr. Tokuju Okano.
“Moreover, the outcome of our work could contribute to the development of treatment strategies for not only arthritis but also other systemic diseases, such as Alzheimer’s disease, which is also related to periodontal pathogenic bacteria.”
More information:
Tokuju Okano et al, Caspase-11 mediated inflammasome activation in macrophages by systemic infection of A. actinomycetemcomitans exacerbates arthritis, International Journal of Oral Science (2024). DOI: 10.1038/s41368-024-00315-x
Citation:
Shedding light on how oral bacteria can aggravate rheumatoid arthritis (2024, September 5)
retrieved 6 September 2024
from https://medicalxpress.com/news/2024-09-oral-bacteria-aggravate-rheumatoid-arthritis.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.