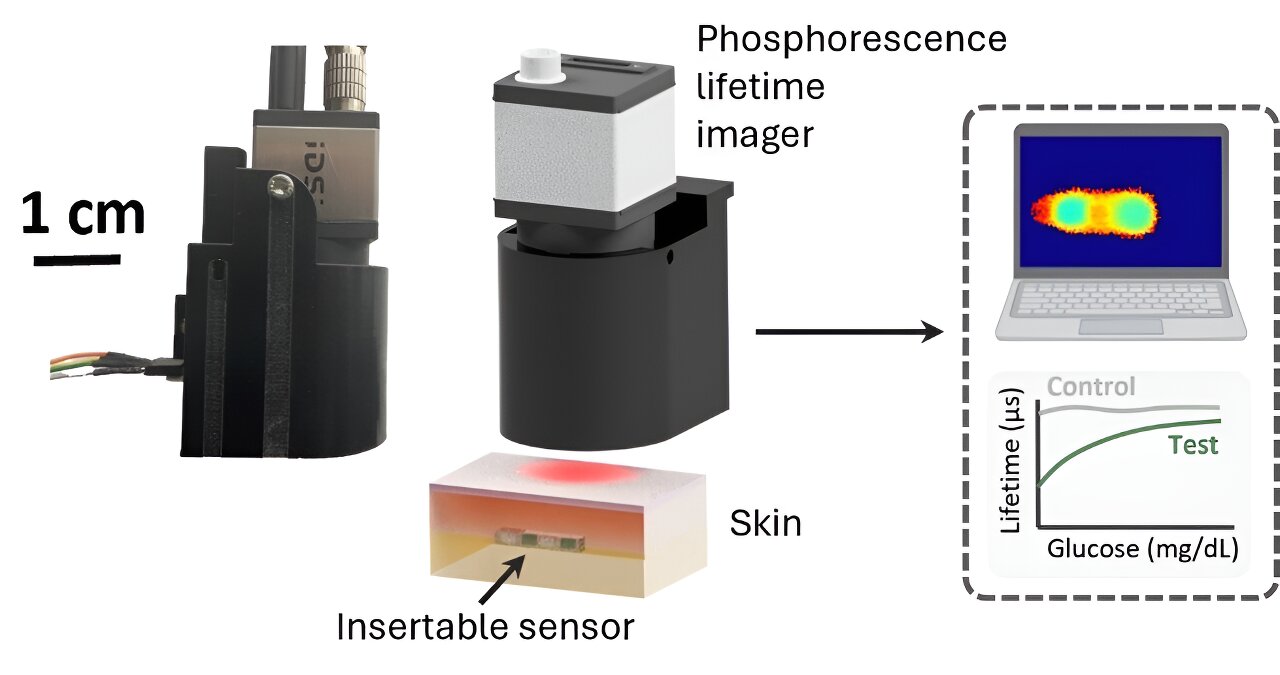
A team of researchers from the University of California, Los Angeles (UCLA), Texas A&M University (TAMU), and Florida International University (FIU) have unveiled a groundbreaking innovation in diabetes management with the development of a novel continuous glucose monitoring (CGM) system.
The new system integrates an insertable glucose biosensor with a phosphorescence lifetime imager (PLI) and advanced machine learning algorithms to offer a more reliable and cost-effective solution for real-time glucose monitoring. The findings are published in the journal ACS Nano.
Diabetes, a chronic condition affecting over 450 million individuals globally, necessitates continuous and precise glucose monitoring to avoid severe health complications. Traditional glucose monitoring methods often require frequent finger pricking, leading to discomfort and reduced adherence to glucose management regimens.
While existing CGM systems have alleviated some of these challenges, they are still associated with high costs, limited sensor lifetimes, and potential tissue irritation due to the invasive nature of electrode insertion.
The new CGM system addresses these limitations by utilizing a biocompatible phosphorescence-based biosensor that is implanted subcutaneously.
Unlike conventional CGMs that rely on electrochemical reactions, this innovative system detects glucose levels through the modulation of phosphorescence signals emitted by the sensor. The emitted signal, which has a significantly longer lifetime compared to tissue autofluorescence, is captured by the compact PLI through the skin, allowing for a non-invasive readout of glucose levels.
One of the most significant advancements of this system is its ability to accurately monitor glucose levels even in the presence of sensor misalignment, a common issue in wearable devices.
The PLI is equipped with a neural network-based model that processes phosphorescence lifetime images to not only infer glucose levels but also detect misalignments, prompting the user to correct the device’s position if necessary. This feature ensures that the glucose readings remain accurate and reliable, even during physical activity or movement, which can often cause misalignments.
In vitro testing demonstrated that the PLI achieved an accuracy of 88.8% in classifying glucose concentrations across normal, low, and high ranges, with an additional capability of identifying misalignments with 100% accuracy.
This robust performance suggests that the system can significantly improve the quality of glucose monitoring, potentially reducing the need for frequent recalibration and offering a more seamless experience for users.
The cost-effectiveness of the new CGM system is another highlight. The insertable biosensor is smaller and more durable than current alternatives, with a stable phosphorescence response lasting up to 12 weeks and enzyme activity maintained for over four weeks.
This extended lifespan reduces the need for frequent sensor replacements, which is a major contributor to the high cost of existing CGM systems. Additionally, the PLI’s compact and portable design, coupled with its affordability, makes it an attractive option for widespread adoption.
Beyond glucose monitoring, the researchers envision broader applications for the PLI system. The imaging platform’s ability to capture phosphorescence signals with high spatial resolution opens up possibilities for multiplexed sensing, where multiple biomarkers can be monitored simultaneously. This capability could revolutionize the field of wearable diagnostics, making the system a versatile tool for real-time health monitoring.
This new CGM system represents a significant step forward in diabetes care, combining cutting-edge technology with patient-centric design. By offering enhanced accuracy, improved comfort, and reduced costs, it holds the potential to transform the way diabetes is managed, ultimately improving the quality of life for millions of individuals worldwide.
The research was conducted by an interdisciplinary team of experts in electrical and computer engineering and biomedical engineering from UCLA, TAMU, and FIU.
More information:
Artem Goncharov et al, Insertable Glucose Sensor Using a Compact and Cost-Effective Phosphorescence Lifetime Imager and Machine Learning, ACS Nano (2024). DOI: 10.1021/acsnano.4c06527
Citation:
New system offers more reliable, cost-effective solution for continuous glucose monitoring (2024, August 14)
retrieved 28 September 2024
from https://medicalxpress.com/news/2024-08-reliable-effective-solution-glucose.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.