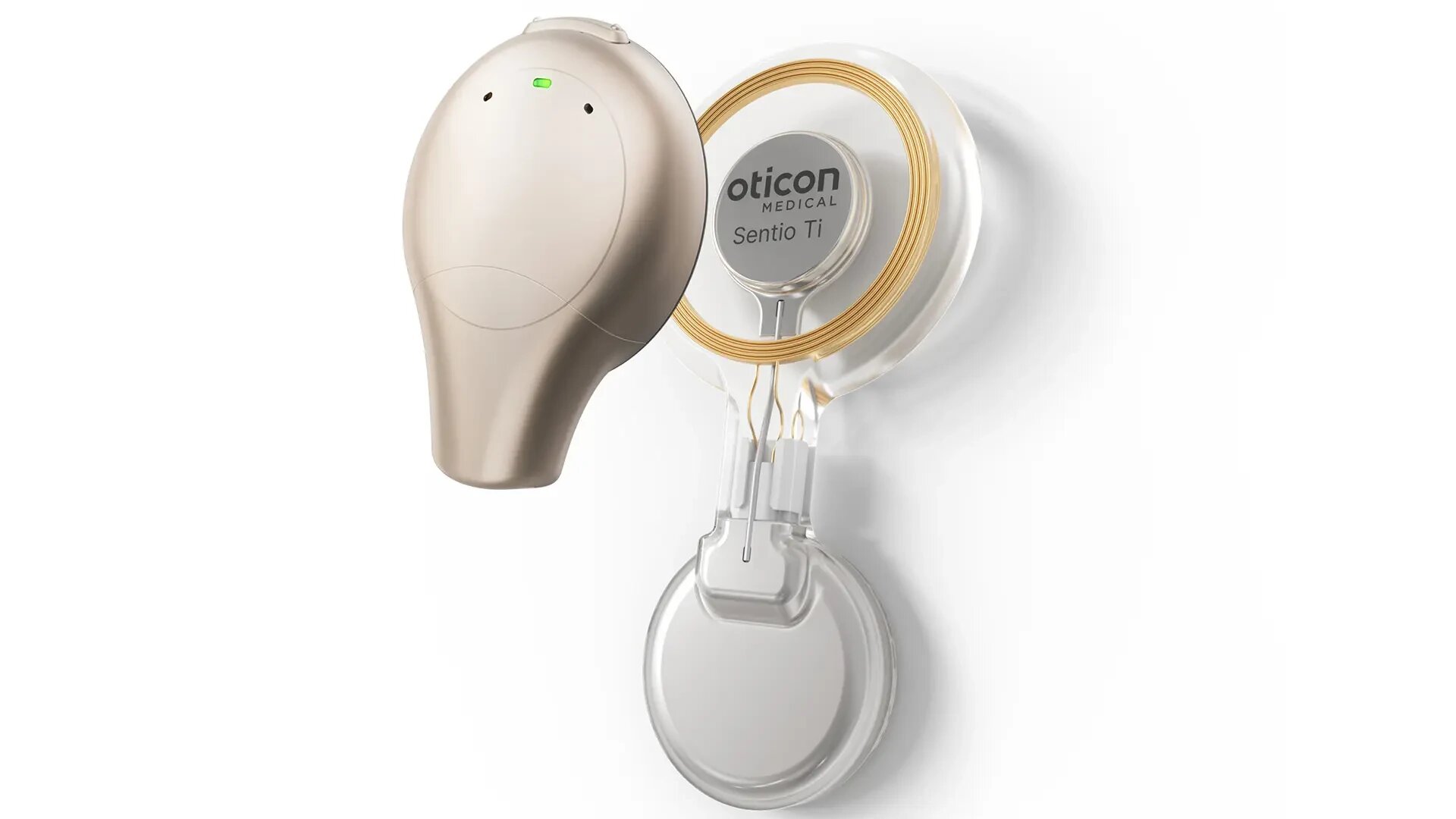
After over two decades of intensive research and development, a new bone conduction implant, the Sentio System, has now been approved for clinical use in both Europe and the United States. This innovative hearing implant originated in a research project at Chalmers University of Technology in collaboration with Sahlgrenska University Hospital and Oticon Medical AB and marks a major progress in medical technology and hearing care.
The Sentio System represents a complete hearing implant developed for patients with damage in the middle ear and ear canal.
“Unlike previous solutions, such as BAHA (Bone Anchored Hearing Aid), which require a permanent skin penetration, Sentio offers an implant with speakers that are placed under the skin. This eliminates the risk of infections associated with permanent skin penetration and is particularly beneficial for younger patients who live an active life and who have higher demands on aesthetics,” says Bo Håkansson, Full Professor in the Biomedical signals and systems research group at the Department of Electrical Engineering at Chalmers.
After 10 years of careful clinical trials, the Sentio System has now been approved under the strict rules for medical devices in Europe and at the same time has been approved by the US Food and Drug Administration, FDA.
“It is very unusual in Sweden that a university-based project reaches commercialization, especially for a so-called Class III device, which includes the highest safety requirements,” says Håkansson.
This breakthrough is expected to have a significant impact on hearing care. The Sentio System eliminates the complications previously associated with bone conduction implants and creates new opportunities for patients with impaired hearing. The researchers behind the project hope that it will not only offer immediate clinical benefit, but also inspire further development in the field, where stronger and more efficient implants can be developed.
This project has also inspired other international bone conduction hearing aid companies that have also developed implants that do not require skin penetration. This project demonstrates the importance of collaboration between academia and industry to take medical device innovations all the way to clinical application.
The Sentio System marks a new era for bone conduction implants and the first patients have already had surgery in both Europe and the U.S.
“The next big step we are working on is to improve the methodology for fitting bone conduction hearing aids to the patient’s specific hearing loss. Until now, subjective and rather imprecise methods have been used, but together with Sahlgrenska Academy and a company in Canada, we have already come a long way towards making the fitting more precise and objective. The latest findings [were] presented at the World Conference in Audiology in Paris at the end of September,” says Håkansson.
Citation:
New bone conduction implant approved in Europe and US (2024, October 7)
retrieved 7 October 2024
from https://medicalxpress.com/news/2024-10-bone-implant-europe.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.