A scratchy, sore throat, a relentless fever, a pounding head and a nasty cough – these symptoms all scream upper respiratory illness. But which one?
Many of the viruses that cause upper respiratory infections such as influenza A or B and the virus that causes COVID-19 all employ similar tactics. They target the same areas in your body – primarily the upper and lower airways – and this shared battleground triggers a similar response from your immune system. Overlapping symptoms – fever, cough, fatigue, aches and pains – make it difficult to determine what may be the underlying cause.
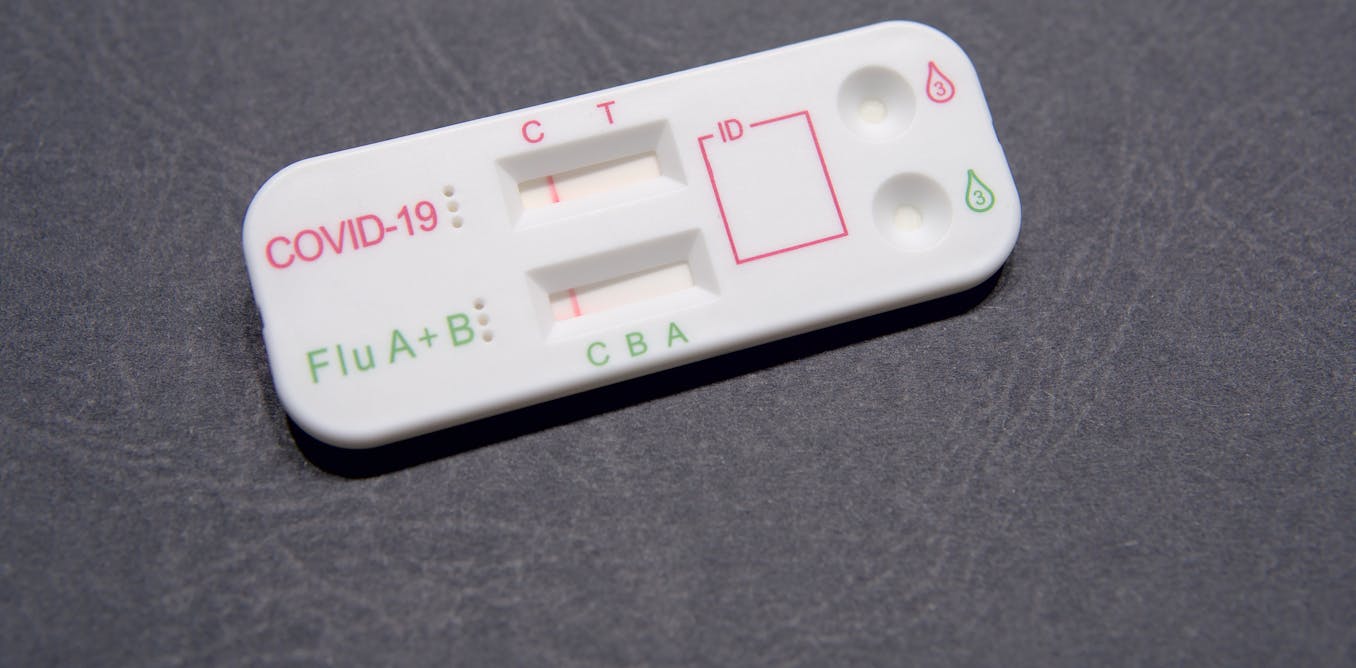
Now, at-home rapid tests can simultaneously determine whether someone has COVID-19 or the flu. Thanks in part to the National Institutes of Health’s Rapid Acceleration of Diagnostics, or RADx, program, the Food and Drug Administration has provided emergency use authorization for seven at-home rapid tests that can distinguish between COVID-19, influenza A and influenza B.
Our team in Atlanta – composed of biomedical engineers, clinicians and researchers at Emory University, Children’s Healthcare of Atlanta and Georgia Institute of Technology – is part of the RADx Test Verification Core. We closely collaborate with other institutions and agencies to determine whether and how well COVID-19 and influenza diagnostics work, effectively testing the tests. Our center has worked with almost every COVID and flu diagnostic on the market, and our data helped inform the instructions you might see in many of the home test kits on the market.
While no test is perfect, to now be able to test for certain viruses at home when symptoms begin can help patients and their doctors come up with appropriate care plans sooner.
A new era of at-home tests
Traditionally, identifying the virus causing upper respiratory illness symptoms required going to a clinic or hospital for a trained medical professional to collect a nasopharyngeal sample. This involves inserting a long, fiber-tipped swab that looks like a skinny Q-tip into one of your nostrils and all the way to the back of your nose and throat to collect virus-containing secretions. The sample is then typically sent to a lab for analysis, which could take hours to days for results.

DuKai/Moment via Getty Images
Thanks to the COVID-19 pandemic, the possibility of using over-the-counter tests to diagnose respiratory illnesses at home became a reality. These tests used a much gentler and less invasive nasal swab and could also be done by anyone, anytime and in their own home. However, these tests were designed to diagnose only COVID-19 and could not distinguish between other types of illnesses.
Since then, researchers have developed over-the-counter multiplex tests that can screen for more than one respiratory infection at once. In 2023, Pfizer’s Lucira test became the first at-home diagnostic test for both COVID-19 and influenza to gain emergency use authorization.
What are multiplex rapid tests?
There are two primary forms of at-home COVID-19 and COVID-19/flu combination tests: molecular tests such as PCR that detect genetic material from the virus, and antigen tests – commonly referred to as rapid tests – that detect proteins called antigens from the virus.
The majority of over-the-counter COVID-19 and COVID-19/flu tests on the market are antigen tests. They detect the presence of antigens in your nasal secretions that act as a biological signature for a specific virus. If viral antigens are present, that means you’re likely infected.
To detect these antigens, rapid tests have paper-like strips coated with specially engineered antibodies that function like a molecular Velcro, sticking only to a specific antigen. Scientists design and manufacture specialized strips to recognize specific viral antigens, like those belonging to influenza A, influenza B or the virus that causes COVID-19.
The antibodies for these viral targets are placed on the strip, and when someone’s nasal sample has viral proteins that are applied to the test strip, a line will appear for that virus in particular.
Advancing rapid antigen tests
Like all technologies, rapid antigen tests have limitations.
Compared with lab-based PCR tests that can detect the presence of small amounts of pathogen by amplifying them, antigen tests are typically less sensitive than PCR and could miss an infection in some cases.
All at-home COVID-19 and COVID-19/flu antigen tests are authorized for repeat use. This means if someone is experiencing symptoms – or has been exposed to someone with COVID-19 but is not experiencing symptoms – and has a negative result for their first test, they should retest 48 hours later.
Another limitation to rapid antigen tests is that currently they are designed to test only for COVID-19, influenza A and influenza B. Currently available over-the-counter tests aren’t able to detect illnesses from pathogens that look like these viruses and cause similar symptoms, such as adenovirus or strep.
Because multiplex texts can detect several different viruses, they can also produce findings that are more complex to interpret than tests for single viruses. This may increase the risk of a patient incorrectly interpreting their results, misreading one infection for another.
Researchers are actively developing even more sophisticated tests that are more sensitive and can simultaneously screen for a wider range of viruses or even bacterial infections. Scientists are also examining the potential of using saliva samples in tests for bacterial or viral infections.
Additionally, scientists are exploring integrating multiplex tests with smartphones for rapid at-home diagnosis and reporting to health care providers. This may increase the accessibility of these tests for people with vision impairment, low dexterity or other challenges with conducting and interpreting at-home tests.
Faster and more accurate diagnoses lead to more targeted and effective treatment plans, potentially reducing unnecessary antibiotic use and improving patient outcomes. The ability to rapidly identify and track outbreaks can also empower public health officials to better mitigate the spread of infectious diseases.