Rutgers Robert Wood Johnson Medical School and collaborating institutions have found that overnutrition leads to insulin resistance and metabolic disorders through increased activity of the sympathetic nervous system (SNS). The study shows that reducing SNS activity can prevent insulin resistance induced by a high-fat diet, suggesting a new understanding of how obesity causes insulin resistance.
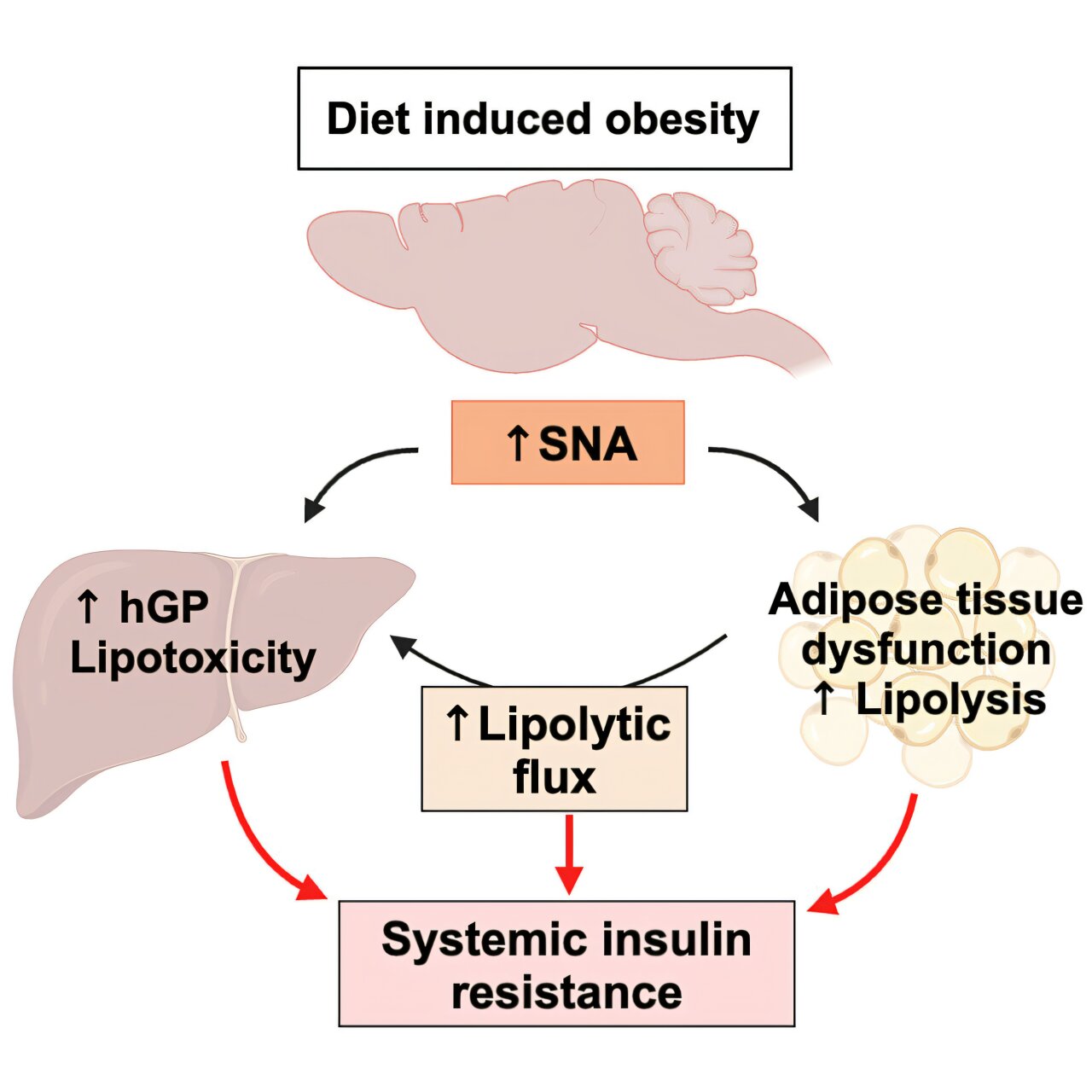
Obesity causes type 2 diabetes and metabolic diseases primarily by inducing insulin resistance. Impaired cellular insulin signaling is the most understood mechanism, but it does not always accompany impaired insulin action, indicating other factors must be involved.
The role of the SNS in obesity is complex and somewhat controversial. Previous studies have reported both increased and decreased SNS activity in obese people.
Overnutrition has been known to rapidly increase plasma norepinephrine (NE) levels, indicating overactivation of the SNS. Methods that directly measure SNS activity, such as nerve recordings and NE turnover, often report increased SNS activity in obesity.
In contrast, studies focusing on adrenergic signaling pathways sometimes report reduced catecholamine responses, interpreted as decreased SNS activity.
This discrepancy may be explained by the development of catecholamine resistance due to chronic sympathetic overactivation, leading to diminished physiological responses despite elevated NE levels.
In a study titled “Overnutrition causes insulin resistance and metabolic disorder through increased sympathetic nervous system activity,” published in Cell Metabolism, the researchers investigated the conflicting reports on SNS activity in obesity.
The researchers utilized a mouse model with inducible and peripherally restricted deletion of the tyrosine hydroxylase gene (THΔper mice). Tyrosine hydroxylase is a required enzyme in synthesizing catecholamines, including NE. By selectively deleting it in peripheral tissues while preserving central nervous system catecholamine levels, the model allowed the study of isolated peripheral SNS activity without affecting central NE functions.
THΔper mice and wild-type littermates were fed either a regular chow diet or a high-fat diet (HFD) for varying durations to simulate short-term and long-term overnutrition.
Short-term overnutrition
Wild-type mice fed an HFD for 3 to 10 days exhibited increased body fat, impaired glucose tolerance and insulin sensitivity compared to regular chow fed mice.
Despite elevated insulin levels, these mice had higher fasting and fed blood glucose levels. Cellular insulin signaling remained intact in HFD-fed mice, suggesting that insulin resistance occurred despite normal insulin signaling pathways.
HFD-fed wild-type mice had elevated plasma NE levels, indicating increased SNS activity. There was an impaired ability of insulin to suppress hormone-sensitive lipase activation in white fat tissue, leading to increased lipolysis and higher plasma glycerol levels.
THΔper mice fed an HFD for up to 14 days were protected from the glucose intolerance and insulin resistance observed in wild-type mice. They maintained normal fasting glucose levels and showed improvement in glucose tolerance tests.
Insulin signaling pathways remained comparable between the THΔper and wild-type mice. Improved insulin sensitivity in THΔper mice was independent of any changes in cellular insulin signaling.
THΔper mice showed a more than 90% reduction in NE levels in peripheral tissues, with no significant change in brain NE levels. They exhibited reduced plasma glycerol levels and improved insulin-mediated suppression of hormone-sensitive lipase phosphorylation in white fatty tissues, indicating better regulation of lipolysis.
Subcutaneous infusion of NE for 14 days in regular chow-fed wild-type mice increased plasma NE levels and impaired insulin action without affecting body weight.
Long-term overnutrition
Wild-type mice fed an HFD for 12 weeks exhibited catecholamine resistance. Nerve recordings confirmed that SNS activity was elevated after 16 weeks of HFD feeding in wild-type mice. After 10 to 12 weeks of HFD feeding, these mice displayed glucose intolerance and elevated levels of NE, epinephrine, and glucagon, indicating increased activation of mechanisms that oppose insulin action.
HFD-induced adipose tissue dysfunction in wild-type mice was characterized by reduced expression of lipogenic enzymes in white fatty tissue, larger adipocyte sizes, and increased markers of inflammation, fibrosis, and senescence.
THΔper mice were protected from developing catecholamine resistance. These mice had significantly lower plasma NE levels after HFD feeding, suggesting reduced peripheral SNS activity. These mice continued to be protected from glucose intolerance despite similar weight gain and adiposity compared to wild-type mice. THΔper mice had significantly lower plasma levels of NE, epinephrine, and glucagon after HFD feeding, indicating reduced activation of these counterregulatory hormones.
THΔper mice were protected from HFD-induced adipose tissue dysfunction. They maintained higher expression of lipogenic enzymes in white fatty tissue, had smaller adipocyte sizes, and exhibited reduced markers of inflammation, fibrosis, and senescence.
The researchers suggest their study is a paradigm shift in understanding obesity-induced insulin resistance. SNS overactivation rather than impaired cellular insulin signaling was the primary driver in the mice obtaining or avoiding insulin resistance.
The implications for type 2 diabetes prevention and treatment could be far-reaching. As with any good research that tells us something we did not know before, more research is needed to see how many new things we can learn from it.
More information:
Kenichi Sakamoto et al, Overnutrition causes insulin resistance and metabolic disorder through increased sympathetic nervous system activity, Cell Metabolism (2024). DOI: 10.1016/j.cmet.2024.09.012
© 2024 Science X Network
Citation:
Insulin resistance caused by sympathetic nervous system over-activation, a paradigm-shifting study finds (2024, November 2)
retrieved 2 November 2024
from https://medicalxpress.com/news/2024-10-insulin-resistance-sympathetic-nervous-paradigm.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.