Researchers at Tel Aviv University cooperated with three major Israeli medical centers to develop a new method for detecting protein aggregation in cells—a hallmark of Parkinson’s disease. The technology can enable diagnosis up to 20 years before the first motor symptoms appear, facilitating treatment or even prevention of the severe disease which is currently incurable.
The novel approach is based on super-resolution microscopy combined with computational analysis, allowing for precise mapping of the aggregates’ molecules and structure. The researchers explain, “Our method can be used to identify early signs and enable preventive treatment in young people at risk for developing Parkinson’s later on in their lives. In the future the technology may also be adapted for early diagnosis of other neurodegenerative diseases, including Alzheimer’s.”
The study was piloted by researchers from the School of Neurobiology, Biochemistry & Biophysics at the Wise Faculty of Life Sciences, the Sagol School of Neuroscience, and the Faculty of Medical and Health Sciences at Tel Aviv University, led by Prof. Uri Ashery and Ph.D. candidate Ofir Sade.
The paper was published in Frontiers in Molecular Neuroscience.
Prof. Ashery says, “Parkinson’s disease is the second most prevalent neurodegenerative disease in the world after Alzheimer’s—with about 8.5 million people with Parkinson’s living worldwide today, and 1,200 new sufferers diagnosed annually in Israel. The debilitating disease is characterized by the destruction of dopaminergic (dopamine producing) neurons in the brain’s Substantia Nigra area.
“Today, diagnosis of Parkinson’s disease is based mainly on clinical symptoms such as tremors or gait dysfunctions, alongside relevant questionnaires. However, these symptoms usually appear at a relatively advanced stage of the disease, when over 50%, and up to 80% of the dopaminergic neurons in the Substantia Nigra are already dead.
“Consequently, available treatments are quite limited in their effect, and usually address only motor problems. In this study we began to develop a research tool to enable diagnosis of Parkinson’s at a much earlier stage, when it is still treatable, and deterioration can be prevented.”
Sade adds, “One known feature of Parkinson’s is cell death resulting from aggregates of the alpha-synuclein protein. The protein begins to aggregate about 15 years before symptoms appear, and cells begin to die 5–10 years before diagnosis is possible with the means available today.
“This means that we have an extensive time window of up to 20 years for diagnosis and prevention, before symptoms appear. If we can identify the process at an early stage, in people who are 30, 40, or 50 years old, we may be able to prevent further protein aggregation and cell death.”
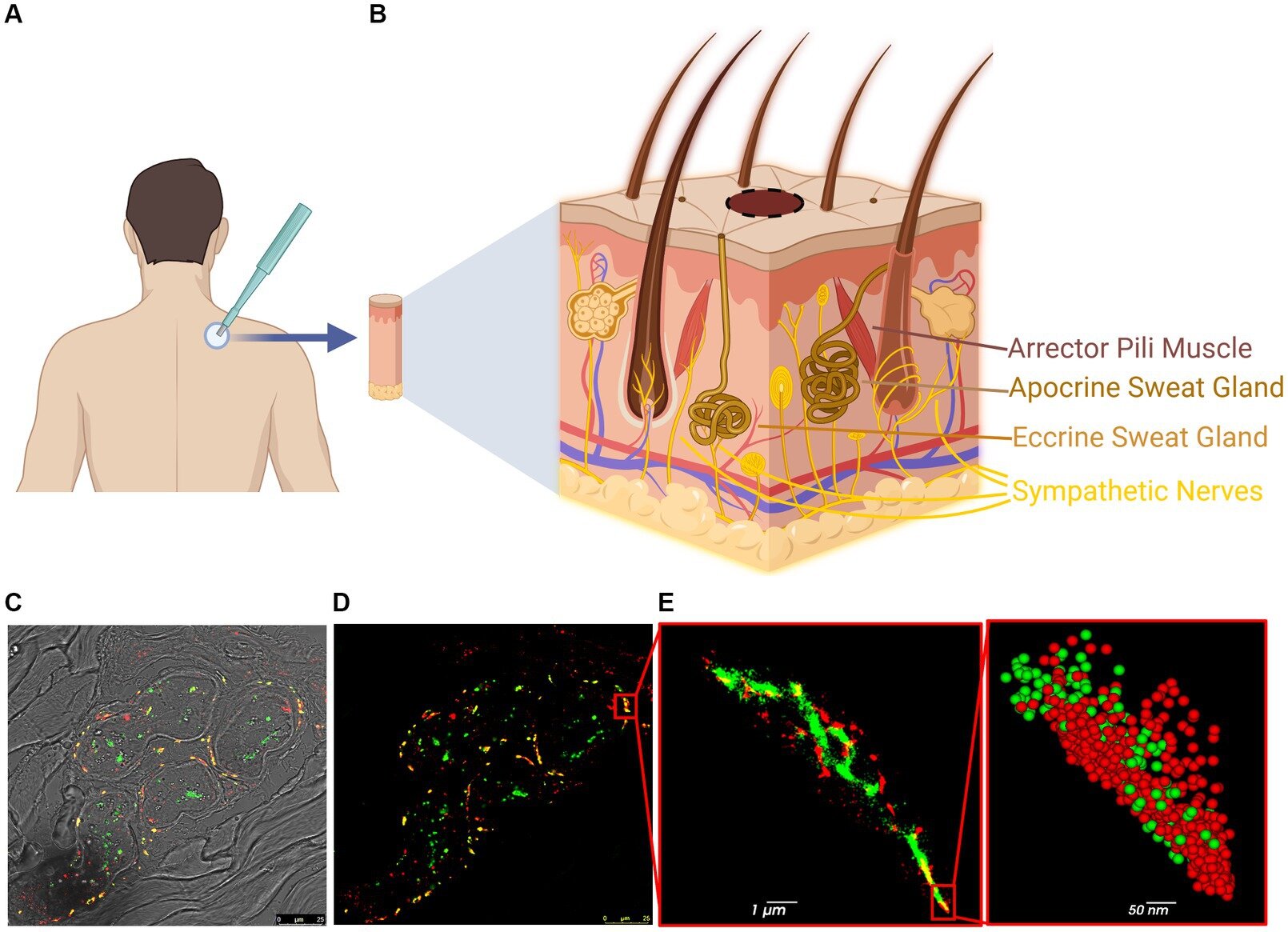
Past studies have shown that alpha-synuclein aggregates form in other parts of the body as well, such as the skin and digestive system. In the current work, the researchers examined skin biopsies from 7 people with and 7 people without Parkinson’s disease, received from the Sheba, Ichilov, and Meir Medical Centers.
Sade notes, “We examined the samples under a unique microscope, applying an innovative technique called super-resolution imaging, combined with advanced computational analysis—enabling us to map the aggregates and distribution of alpha-synuclein molecules. As expected, we found more protein aggregates in people with Parkinson’s compared to people without the disease. We also identified damage to nerve cells in the skin, in areas with a large concentration of the pathological protein.”
With proof of concept obtained through the study, the researchers now plan to expand their work. In the next phase, they will increase the number of samples to 90—45 from healthy subjects and 45 from people without Parkinson’s disease—in order to identify differences between the two groups.
Sade says, “We intend to pinpoint the exact juncture at which a normal quantity of proteins turns into a pathological aggregate. In addition, we will collaborate with Prof. Lior Wolf of TAU’s School of Computer Science to develop a machine learning algorithm that will identify correlations between results of motor and cognitive tests and our findings under the microscope. Using this algorithm, we will be able to predict the future development and severity of various pathologies.”
Prof. Ashery says, “In this study we identified differences between tissues taken from people with and without Parkinson’s disease, using super-resolution microscopy and computational analysis. In future studies we will increase the number of samples and develop a machine learning algorithm to spot relatively young individuals at risk for Parkinson’s.
“Our main target population are relatives of Parkinson’s patients who carry mutations that increase the risk for the disease. Specifically, we place emphasis on two mutations known to be widespread among Ashkenazi Jews. A clinical trial is already underway to test a drug expected to hinder the formation of the aggregates that cause Parkinson’s disease. We hope that in coming years it will be possible to offer preventive treatments, while tracking the effects of medications under the microscope.
“It is important to note that the method we have developed can also be suitable for early diagnosis of other neurodegenerative diseases associated with protein aggregates in neurons, including Alzheimer’s.”
Other study authors include Prof. Anat Mirelman, Prof. Avner Thaler, Prof. Nir Giladi, Prof. Roy Alcalay, Prof. Sharon Hassin, Prof. Nirit Lev, Dr. Irit Gottfried, Dr. Dana Bar-On, Dr. Meir Kestenbaum, Dr. Saar Anis, Dr. Shimon Shahar, Daphna Fischel, Dr. Noa Barak-Broner, Shir Halevi, and Dr. Aviv Gour—all from Tel Aviv University, with some also affiliated with the Tel Aviv Sourasky (Ichilov), Sheba, or Meir Medical Centers. Researchers from Germany and the U.S. also contributed to the study.
More information:
Ofir Sade et al, A novel super-resolution microscopy platform for cutaneous alpha-synuclein detection in Parkinson’s disease, Frontiers in Molecular Neuroscience (2024). DOI: 10.3389/fnmol.2024.1431549
Citation:
Innovative technology may enable early diagnosis of Parkinson’s disease (2024, September 16)
retrieved 21 September 2024
from https://medicalxpress.com/news/2024-09-technology-enable-early-diagnosis-parkinson.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.