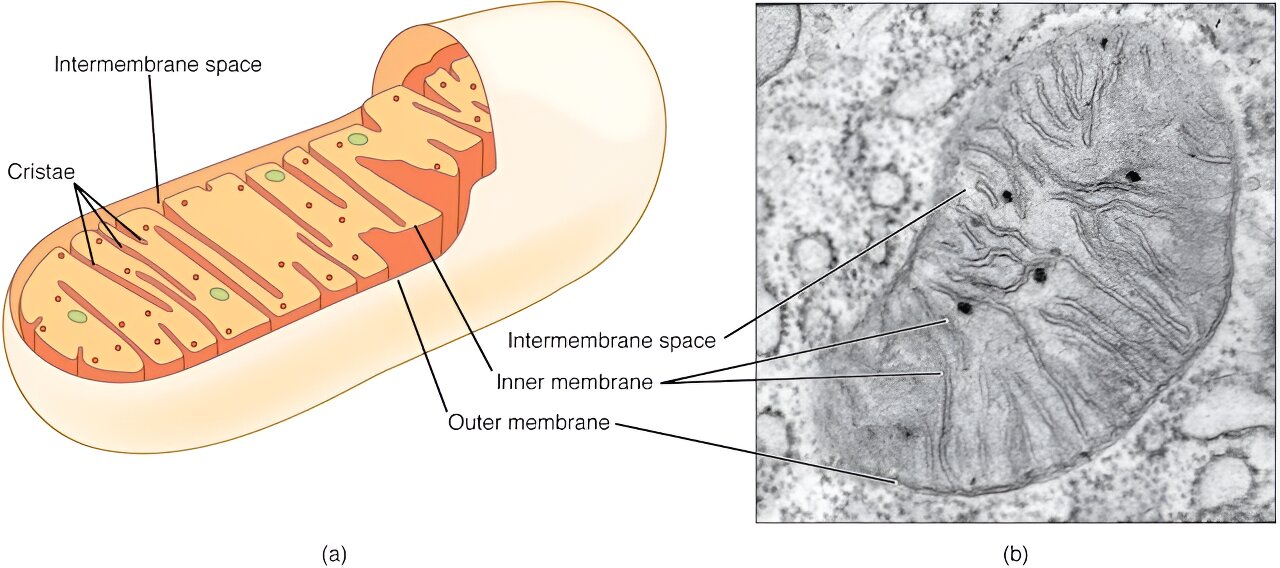
A pilot trial by investigators from Brigham and Women’s Hospital, a founding member of the Mass General Brigham healthcare system, tested the nasal administration of the drug Foralumab, an anti-CD3 monoclonal antibody. Investigators found evidence that the drug dampened the inflammatory T cell response and decreased lung inflammation in patients with COVID-19. Further analysis showed the same gene expression modulation in patients with multiple sclerosis, who experienced decreased brain inflammation, suggesting that Foralumab could be used to treat other diseases. Their results are published in the Proceedings of the National Academy of Sciences.
“We discovered a way to shut down inflammation not only seen in COVID-19, but also in a patient with multiple sclerosis as well as in healthy patients,” said lead author Thais Moreira, PhD, an assistant scientist at the Ann Romney Center for Neurologic Diseases at BWH and an instructor in Neurology at Harvard Medical School. “This is very exciting because not only does our study suggest that this new monoclonal antibody drug is safe and can modulate the immune system without major side effects, but it can also decrease inflammation in multiple realms, so it may be useful for treating other diseases.”
“Inflammation is a major cause of many diseases,” said senior author Howard Weiner, MD, founder and director of the Brigham Multiple Sclerosis Center and co-director of the Ann Romney Center for Neurologic Diseases. “Our center has spent decades looking for novel ways to treat disease where there is abnormal inflammation in a way that is safe and effective.”
In both COVID-19 and multiple sclerosis, the immune system is overactive. Foralumab, manufactured by Tiziana Life Sciences, is a drug that stimulates regulatory T cells of the immune system, or anti-inflammatory cells, resulting in decreased inflammation. This contrasts with other monoclonal antibodies previously given to treat or prevent symptoms of COVID-19 (such as Evusheld) that target the SARS-CoV-2 spike protein, which only had activity against specific variants and subvariants.
In 2020, Moreira traveled to Brazil to carry out this study of Foralumab, which was given nasally to 39 patients with mild-to-moderate COVID-19 infections. Blood analysis showed signs that the patients who received 100ug of Foralumab each day for 10 days experienced less lung inflammation than their counterparts who did not. These findings led the team to carry out a sophisticated gene expression analysis to see how Foralumab was working to modulate the immune response in order to reduce inflammation. This revealed a pattern of three specific genes (NKG7, TGF beB1, and GIMAP7) involved with the anti-inflammatory effects of the drug, not only in the COVID-19 patients from the 2020 study, but also in a patient with multiple sclerosis at Brigham and Women’s Hospital and in healthy volunteers as well.
“This is the first nasal monoclonal antibody — other monoclonal antibody treatments were delivered intravenously and are no longer given as treatment because they are not effective against currently circulating viral variants,” said Weiner. “Based on our studies, we expect that Foralumab may work on all variants as it acts on immune effects. This is promising for other diseases because it works on inflammation which is a major driver of many diseases.”
Given the limitations of the small sample sizes studied, the team is moving ahead with a placebo-controlled double-blind trial in progressive multiple sclerosis as well as planning a new trial to study long COVID.
“Moreira’s discovery of a common pathway and mechanism by which Foralumab modulates the immune system gives us a biomarker that we can use to monitor treatment,” said Weiner. “Next, we are going to look at studying the use of Foralumab in long COVID, larger studies of multiple sclerosis, and other diseases such as Alzheimer’s disease.”
Disclosures: H.L.W. is chair of the Scientific Advisory Board of Tiziana and received consulting fees and stock options from the company. T.C. is a member of the scientific advisory board and serves as a consultant to Tiziana Life Sciences. CMB-A serves as a consultant to Tiziana Life Sciences. T.G.M. and K.T.F.M. received consultation fees from Tiziana Life Sciences to monitor the clinical trials in which samples were used in this present work. In addition to providing Foralumab, Tiziana Life Sciences also provided financial assistance to the trial and immunological studies but did not participate in statistical analysis or data interpretation.
Funding: This study was funded by the Ann Romney Center for Neurological Diseases at Brigham and Women’s Hospital.