Using a novel stem cell platform, a team of MUSC researchers has identified a pathway that could be targeted by drugs to reduce fat accumulation in patients with a common form of fatty liver disease known as metabolic dysfunction-associated steatotic liver disease, or MASLD. The MUSC team was led by Stephen Duncan, Ph.D., SmartState Endowed Chair in Regenerative Medicine at MUSC, and Caren Doueiry, an M.D., Ph.D. candidate in Duncan’s laboratory.
The team reports its findings in the International Journal of Molecular Sciences.
Almost a quarter of Americans have MASLD, formerly known as non-alcoholic fatty liver disease. It is estimated that three in four people who are overweight and as many as two in three patients with type 2 diabetes have the disease. In MASLD, fat accumulates over time in the liver, leading to fibrosis, or scarring of the liver, as well as cancer. In fact, MASLD is the leading cause of hepatocellular carcinoma, the most common primary liver cancer in adults.
Understanding MASLD using stem cells
Stem cells are a group of cells that have not yet determined what types of cells they will become. During early development, they respond to signals in their environment that guide them to differentiate into specific cell types, such as liver, nerve or muscle cells.
Scientists have developed technologies that enable them to take blood samples from patients and erase the specialized markers that make them blood cells. These reprogrammed cells are called induced pluripotent stem cells (iPSCs), and they serve as a blank canvas for scientists, who can instruct them to become any type of cell they wish to study. This technology allows these cells to organize into complex tissues and organs, providing a deeper understanding of the human body.
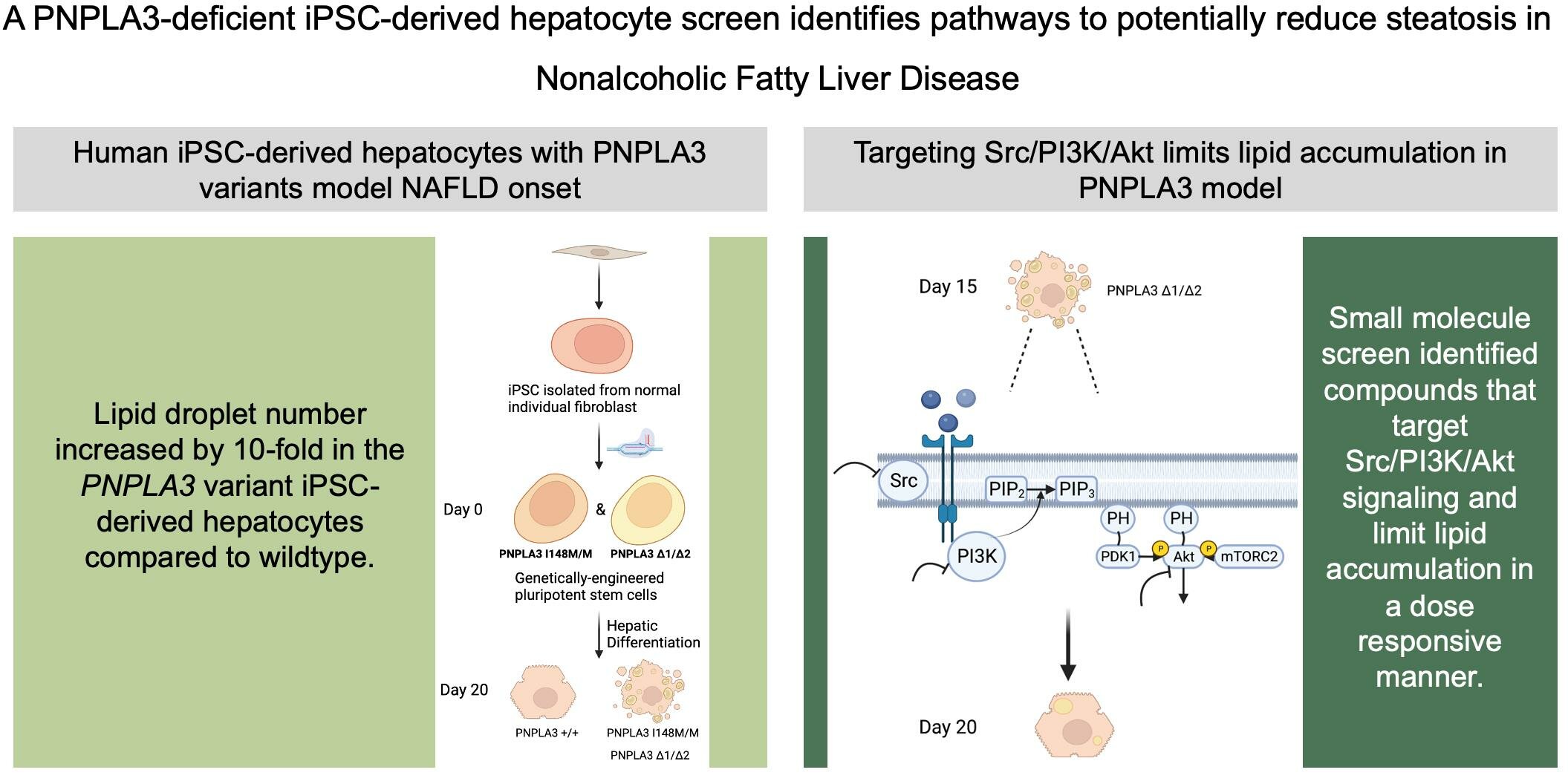
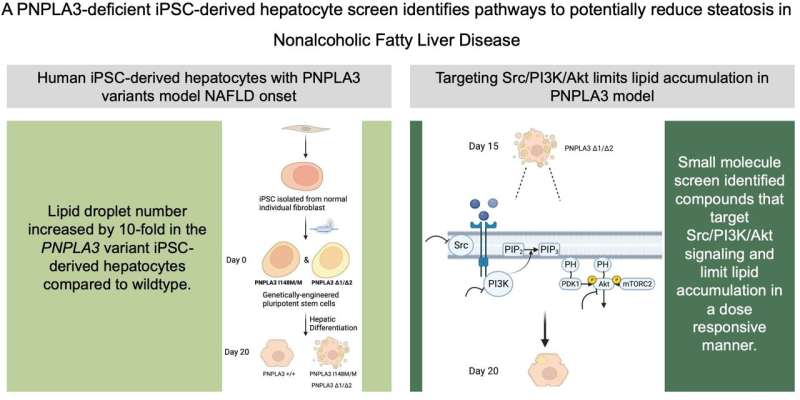
Doueiry used CRISPR, a gene-editing tool, to engineer a line of iPSCs with a mutation in the PNPLA3 gene that is common in patients with MASLD. CRISPR stands for clustered regularly interspaced short palindromic repeats.
“A lot of patients with fatty liver have this mutation, and we don’t know what it’s doing or why it’s increasing susceptibility to fatty liver,” said Doueiry, lead author of the article.
To learn more about the mutation’s role in MASLD, Doueiry induced the iPSCs carrying the mutation to become liver cells. She observed that the mutated liver cells had higher levels of fat accumulation. This finding links this common genetic mutation to a key characteristic of MASLD, showing that it plays an important role in regulating fat accumulation in the liver.
Doueiry is most excited that she now has a model that mirrors the human disease right in a cell culture plate.
“I knew that the mutation in humans was causing the fat accumulation, but I had no idea what I was going to see in the plate,” she said. “Looking at the liver cells carrying the genetic mutation for the first time was so exciting because I knew we had a model that we could use to find some answers.”
Searching for compounds to reduce fat build-up in the liver
No current pharmaceutical treatments target the excess fat accumulation that leads to MASLD. To look for potential contenders, Doueiry screened 1,100 small molecules from a library of compounds to see which ones reduced fat accumulation in her genetically modified liver cells. After a series of screens, Doueiry was able to identify five compounds that more than halved the number of fat droplets on the treated liver cells.
Surprisingly, these compounds all interacted with proteins in the same cellular pathway. Even more remarkably, this cellular pathway is known to regulate cell growth and is commonly targeted by cancer therapeutics to stop tumor development. Several approved cancer drugs that inhibit proteins in this pathway are already being administered to patients. When Doueiry used cancer drugs to treat her genetically modified liver cells, fat accumulation dramatically decreased, just as it had with the five compounds she initially discovered.
“With these results, we knew we weren’t just looking at random molecules doing something. The fact that they all connected through a pathway showed us that we were onto something,” she explained.
Doueiry was even able to use her model to determine the appropriate drug dosage needed to achieve this therapeutic effect with minimal unwanted effects. She discovered that a low dose of the inhibitor was sufficient to lower fat accumulation in liver cells lacking the mutation, with little effect on cell viability, promising findings for future clinical studies.
In short, Doueiry’s iPSC disease model not only mimicked the disease in the laboratory but also provided a better sense of how human cells would respond to treatment. It showed that low dosages of selected pathway inhibitors achieved a good response, with few unwanted effects.
Duncan is excited about the potential of this new model.
“Caren’s study has shown that human stem cell-derived liver cells with MASLD mutations can be used effectively to identify pathways that can be targeted by drugs to reduce fat levels in the liver,” said Duncan.
What does this mean for patients with MASLD?
This study’s finding suggests that MASLD, particularly when caused by this common genetic mutation, could one day be a treatable condition, possibly using repurposed already-approved drugs.
Though much work remains before a pharmaceutical treatment reaches the clinic, Duncan and Doueiry’s work inspires hope—not just for patients with MASLD but also for the potential of iPSCs to serve as models for screening therapeutics for other genetically linked diseases.
More information:
Caren Doueiry et al, A PNPLA3-Deficient iPSC-Derived Hepatocyte Screen Identifies Pathways to Potentially Reduce Steatosis in Metabolic Dysfunction-Associated Fatty Liver Disease, International Journal of Molecular Sciences (2024). DOI: 10.3390/ijms25137277
Citation:
Finding the right pathway to reduce fat accumulation in the liver (2024, September 10)
retrieved 13 September 2024
from https://medicalxpress.com/news/2024-09-pathway-fat-accumulation-liver.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.