An estimated one-third of patients with major depressive disorder have treatment-resistant depression (TRD), characterized by an inadequate response to two or more oral antidepressants (OADs).
Three international clinical trials have shown that esketamine (an intranasally delivered, specialized form of the anesthetic and antidepressant ketamine) can improve depression symptoms in patients with TRD when taken with a newly initiated OAD. However, whether these effects persisted in patient-reported measures was unknown.
To answer this question, researchers led by Madhukar Trivedi, M.D., Professor of Psychiatry and Director of the Center for Depression Research and Clinical Care in the Peter O’Donnell Jr. Brain Institute at UT Southwestern, analyzed data from the clinical trials.
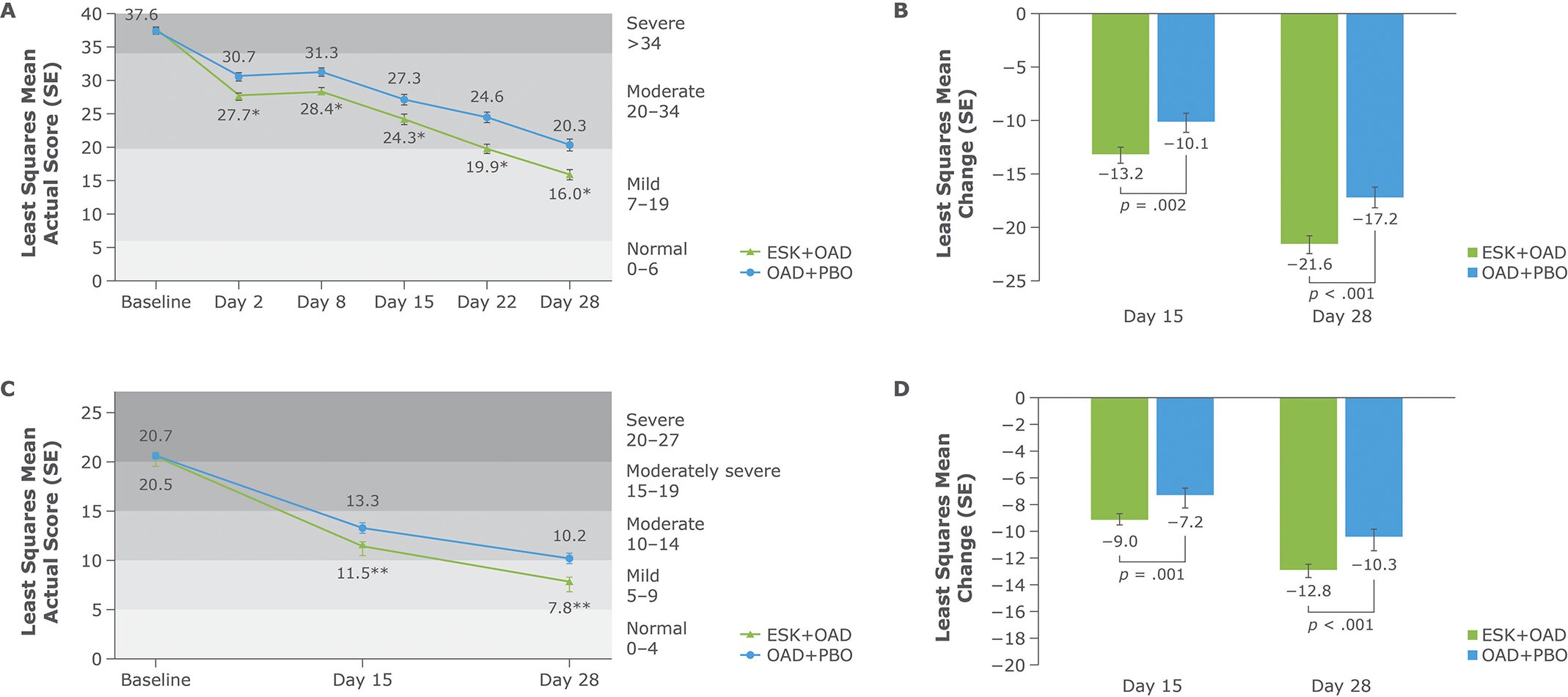
Results, published in CNS Spectrums, showed a significant improvement in symptoms for those taking a new OAD with esketamine compared with a placebo nasal spray based on responses to the nine-item Patient Health Questionnaire, a validated measure of depression severity.
Participants were also significantly more likely to achieve remission from TRD with esketamine than with the placebo. These results show a strong correlation between patient- and physician-reported measures and the promise of esketamine treatment for TRD.
More information:
Jennifer Kern Sliwa et al, Effects of esketamine nasal spray on depressive symptom severity in adults with treatment-resistant depression and associations between the Montgomery–Åsberg Depression Rating Scale and the 9-item Patient Health Questionnaire, CNS Spectrums (2024). DOI: 10.1017/S1092852924000105
Citation:
Esketamine shows promise for treatment-resistant depression (2024, September 27)
retrieved 28 September 2024
from https://medicalxpress.com/news/2024-09-esketamine-treatment-resistant-depression.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.