Researchers from the University of Oxford have identified a key biochemical mechanism relevant to the development of Huntington’s disease. This discovery opens up the possibility of studying the disease before its clinical onset and eventually stopping its progression.
The study, published in Nature Metabolism, has shown for the first time the biochemical change responsible for the development of Huntington’s disease, and how blocking this change stopped disease progression.
Huntington’s disease (HD) is an inherited condition that stops parts of the brain from working properly, leading to mental and physical decline that slowly worsens over time. The symptoms usually begin to appear after the age of 30 years and are fatal, but this can be after a period of up to 20 years, during which they worsen.
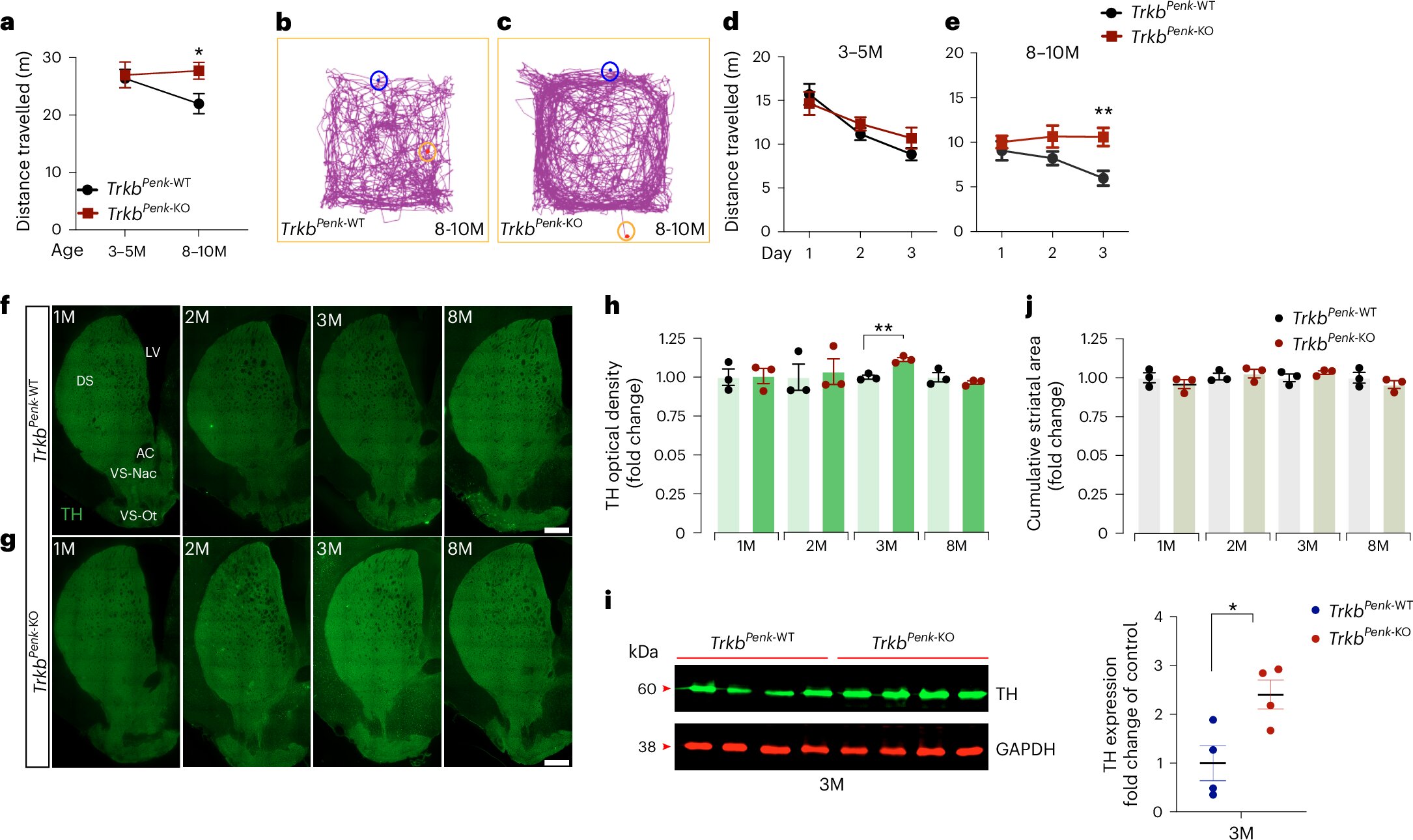
The study explores how an early change described in the brain of HD patients in the early 1980s could lead to Huntington’s disease onset. The researchers identified that problems with specific neurons in the brain, called indirect pathway spiny projection neurons (iSPNs), which are the initially affected cells in HD, may trigger an imbalance in dopamine levels upon missing an important signaling derived from the activation of the neurotrophin receptor TrkB. This imbalance is linked to early symptoms of the disease, such as abnormal, involuntary movements.
First, the researchers looked at mice that lacked normal function in these iSPNs due to disrupted TrkB neurotrophin signaling and noticed that they showed increased levels of dopamine in the brain, leading to hyperactivity. This change occurred before noticeable symptoms appeared, suggesting that these early alterations may contribute significantly to HD progression.
The researchers also found that a protein called GSTO2, an enzyme that is part of the glutathione metabolism, plays an important role in regulating dopamine levels. By selectively reducing the activity of this protein in mice, the researchers were able to prevent dopamine and energy metabolism dysfunction, arresting the onset of motor symptoms in mice.
Importantly, this enzyme shows similar dysregulation in a rat model of HD and some rare brains of asymptomatic HD patients, confirming its putative relevance to the development of the disorder.
The study’s lead author, Liliana Minichiello, Professor of Cellular and Molecular Neuroscience at Oxford’s Department of Pharmacology, said, “The big problem with Huntington’s disease is that by the time that symptoms develop much of the damage has already been done, and therefore, it is fundamental that we understand the changes that occur before the disorder develops if we are to develop effective therapeutics.”
“This research marks the first time that we have been able to identify a specific chemical change that is unique to the development of Huntington’s disease, which opens the possibility of developing new tests to study the early changes of the disease before irreversible damage occurs.
“Understanding these early changes provides crucial insights into how Huntington’s Disease develops, and this knowledge could help develop preventive therapies to maintain dopamine balance and delay or halt disease progression.”
Dr. Yaseen Malik (Department of Pharmacology, Oxford University), first author of the paper, said, “Despite our significant understanding of its pathophysiology, HD remains without a cure, which underscores the necessity of delivering diagnostic and therapeutic interventions prior to the onset of symptoms, and this study is a step in that direction.”
More information:
Mohd Yaseen Malik et al, Impaired striatal glutathione–ascorbate metabolism induces transient dopamine increase and motor dysfunction, Nature Metabolism (2024). DOI: 10.1038/s42255-024-01155-z
Citation:
Discovery of key mechanism in Huntington’s disease could pave the way for early detection and treatment (2024, October 28)
retrieved 28 October 2024
from https://medicalxpress.com/news/2024-10-discovery-key-mechanism-huntington-disease.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.