Tokyo Medical and Dental University (TMDU) researchers have developed a technique that allows a detailed analysis of periodontitis development over time.
Periodontal disease, represented by periodontitis, is the leading cause of tooth loss and affects close to one in five adults worldwide. In most cases, this condition occurs as a result of an inflammatory response to bacterial infection of the tissue around teeth.
As the condition worsens, the gums begin to pull away, exposing teeth roots and bone. Notably, the incidence of periodontitis becomes more prevalent with age and with populations worldwide living longer, developing a solid understanding of its underlying causes and progression is important.
In a study published in Nature Communications on March 28 2024, researchers from TMDU found a way to achieve this by improving upon a widely used animal model to study periodontitis.
Studying periodontitis directly in humans is challenging. As a result, scientists often resort to animal models for preclinical research. For instance, the “mouse ligature-induced periodontitis model,” since its inception in 2012, has enabled researchers to study the cellular mechanisms underlying this condition.
Simply put, with this model, periodontal disease is artificially induced by ligating silk threads onto the molars of mice models, which induces plaque accumulation. While convenient and effective, this model, however, fails to capture the complete picture of periodontitis.
“Even though the periodontal tissue is composed of gingiva, periodontal ligament, alveolar bone, and cementum, analyses are usually performed exclusively on gingival samples due to technical and quantitative limitations,” remarks lead author Mr. Anhao Liu. “This sampling strategy limits the conclusions that may be drawn from these studies, so methods that allow for the simultaneous analysis of all tissue components are needed.”
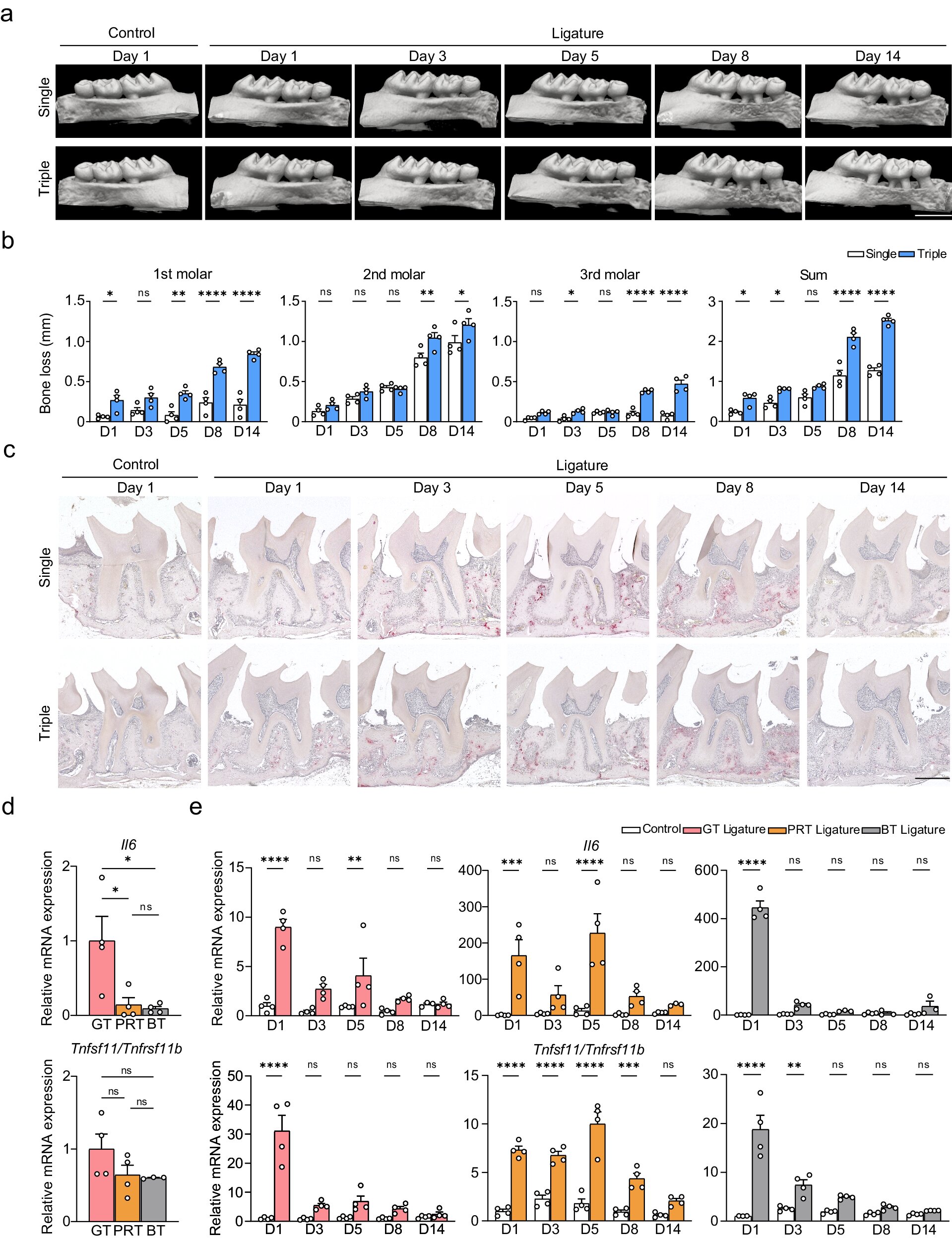
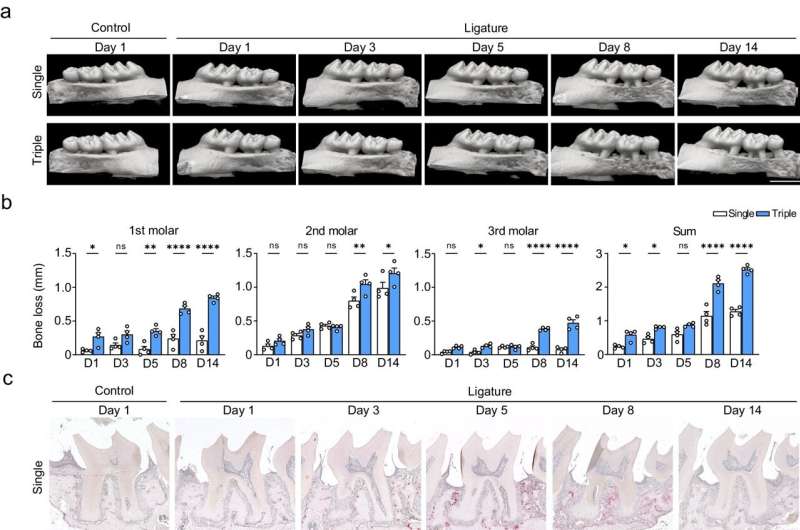
To address this limitation, the research team developed a modified ligature-induced periodontitis model. Instead of the classic single ligature, they used a triple ligature approach on the upper left molar of male mice. This strategy expanded the range of bone loss without causing severe bone destruction around the second molar, increasing the yield of the different types of periodontal tissue.
“We isolated the three main tissue types and evaluated the RNA yield between the two models. The results showed that the triple-ligature model effectively increased the yield, achieving four times the yield of normal peri-root tissue and supporting the high-resolution analysis of different tissue types,” explains senior author Dr. Mikihito Hayashi.
After confirming the efficacy of their modified model, the researchers proceeded to investigate the effects of periodontitis on gene expression among the different tissue types over time, focusing on genes related to inflammation and osteoclast differentiation.
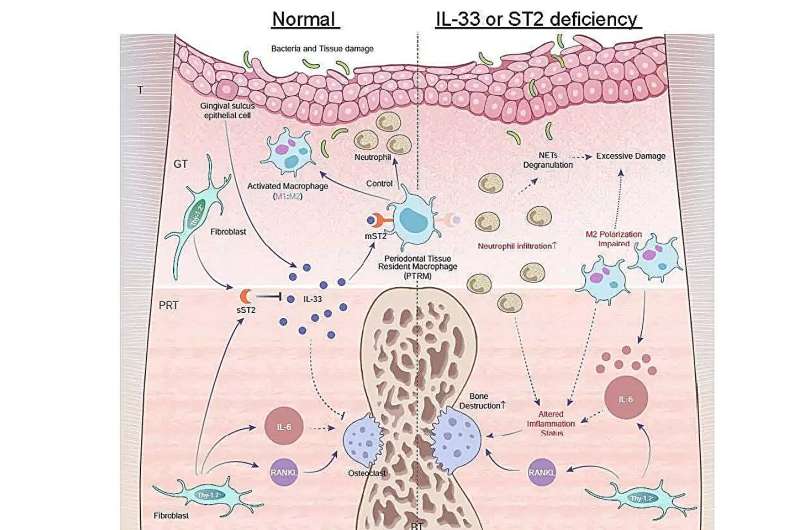
One of their main findings was that the expression of the Il1rl1 gene was markedly higher in peri-root tissue five days after ligation. This gene encodes the protein ST2 in both receptor and decoy isoform, which binds to a cytokine called IL-33 that is involved in inflammatory and immunoregulation processes.
To gain further insights into the role of this gene, the team induced periodontitis in genetically modified mice that lacked the Il1rl1 or Il33 genes. These mice exhibited accelerated inflammatory bone destruction, highlighting the protective role of the IL-33/ST2 pathway. Further analysis of cells containing the ST2 protein in its receptor form, mST2, revealed that most of them were of macrophage lineage.
“Macrophages are typically classified into two main types, pro-inflammatory and anti-inflammatory, based on their activation process. We found that mST2-expressing cells were unique in that they expressed some markers of both types of macrophages simultaneously,” comments senior author Dr. Takanori Iwata. “These cells were present in the peri-root tissue before inflammation was triggered, so we named them ‘periodontal tissue-resident macrophages.'”
Together, the findings of this study showcase the power of this modified animal model to study the full scope of periodontitis in greater detail, right down to the biomolecular level.
“We suggest the possibility that a novel IL-33/ST2 molecular pathway regulating inflammation and bone destruction in periodontal disease, alongside specific macrophages in peri-root tissue, is deeply involved in periodontal disease. This will hopefully lead to the development of new treatment strategies and prevention methods,” concludes senior author Dr. Tomoki Nakashima.
More information:
Anhao Liu et al, The IL-33/ST2 axis is protective against acute inflammation during the course of periodontitis, Nature Communications (2024). DOI: 10.1038/s41467-024-46746-2
Citation:
Clarifying the cellular mechanisms underlying periodontitis with an improved animal model (2024, May 21)
retrieved 13 September 2024
from https://medicalxpress.com/news/2024-05-cellular-mechanisms-underlying-periodontitis-animal.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.