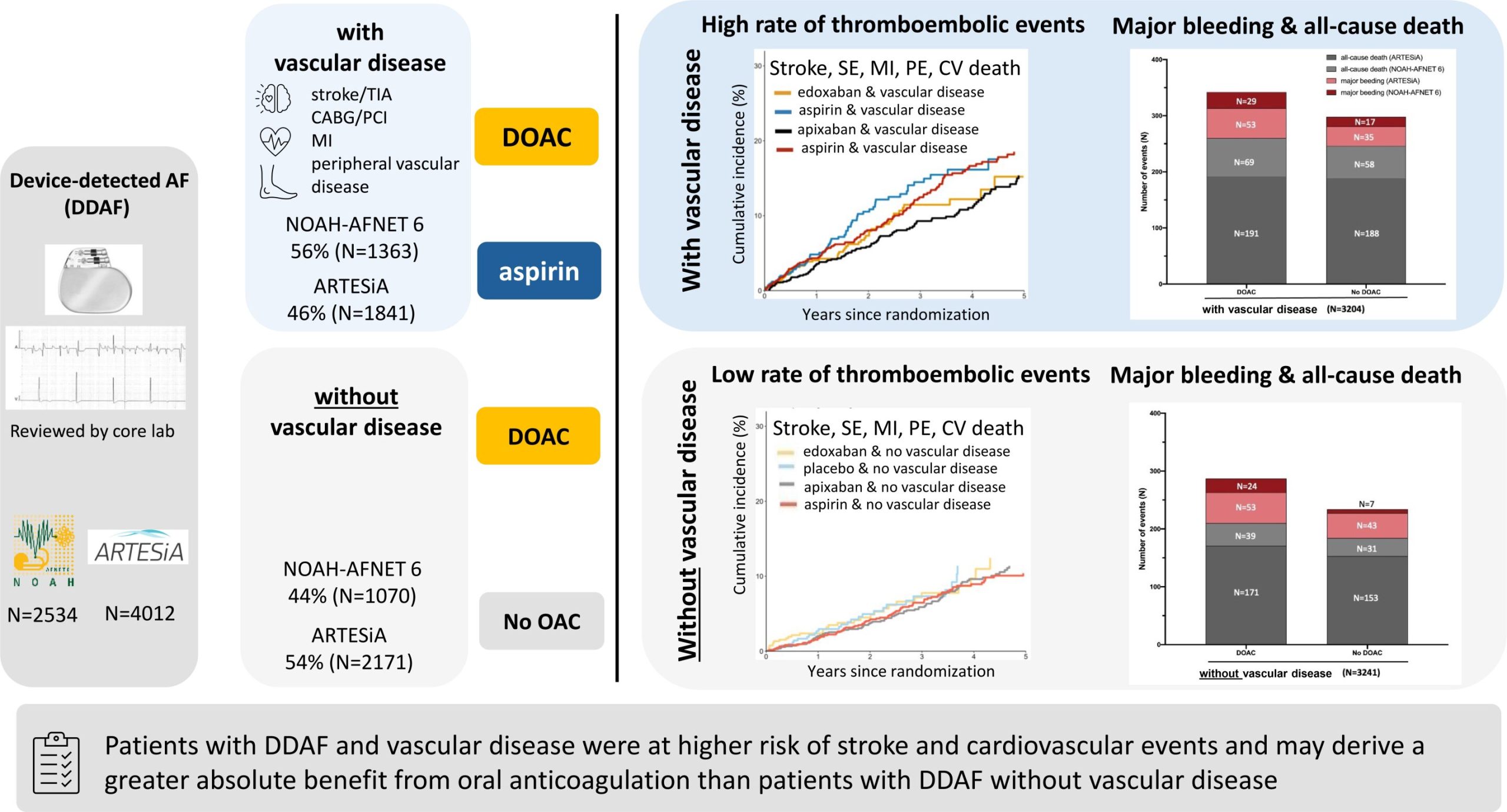
A combined subgroup analysis of the similar trials NOAH—AFNET 6 and ARTESiA has revealed that patients with device-detected atrial fibrillation and concomitant vascular disease are at higher risk of stroke and cardiovascular events and may derive a greater benefit from oral anticoagulation than those without vascular disease.
The finding was presented by AFNET Steering Committee member Prof. Renate Schnabel, University Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany, at the annual congress of the European Society of Cardiology (ESC) in London on Sept. 2, 2024 and published in the European Heart Journal.
Device-detected atrial fibrillation (DDAF) are short and typically rare episodes of atrial fibrillation (AF) detected by pacemakers, defibrillators, or implanted loop recorders. Device-detected atrial fibrillation is found in every fifth patient with a cardiac implanted electronic device. Device-detected atrial fibrillation can lead to stroke, but the stroke risk in patients with device-detected atrial fibrillation appears lower than the stroke risk in patients with ECG-documented atrial fibrillation (1%/year).
Two recent trials, NOAH—AFNET 6 and ARTESiA, assessed the benefit of anticoagulation in patients with DDAF and stroke risk factors, but without ECG-documented AF. In both trials, patients were randomized either to anticoagulation (edoxaban in NOAH—AFNET 6 and apixaban in ARTESiA) or no anticoagulation in order to compare efficacy and safety outcomes in both groups.
NOAH—AFNET 6 (Non vitamin K antagonist Oral anticoagulants in patients with Atrial High-rate episodes), an investigator-initiated trial conducted by the AFNET, was terminated early due to an expected increase in bleeding events in patients with device-detected atrial fibrillation while the stroke preventing effect was smaller than expected. The weak effects of anticoagulation are also found in several subgroups including patients with long episodes of device-detected AF, patients with a high comorbidity burden, and patients with prior stroke.
ARTESiA (Apixaban for the Reduction of Thrombo-Embolism in Patients with Device-Detected Sub-Clinical Atrial Fibrillation) confirmed the low rate of stroke in patients with DDAF and demonstrated a small stroke-reducing effect of anticoagulation. A meta-analysis of NOAH—AFNET 6 and ARTESiA confirmed an increase in bleeding and detected a small reduction in ischemic strokes with anticoagulation.
Prof. Schnabel, leading investigator of the combined NOAH—AFNET 6 / ARTESiA sub-analysis presented now at the ESC congress, explained the background of this research, saying, “About half of patients with device-detected atrial fibrillation have concomitant vascular disease, i.e. prior stroke or transient ischemic attack (TIA), coronary or peripheral vascular disease. The main goal of our pre-specified subgroup analysis was to assess whether vascular disease affects the efficacy and safety of oral anticoagulation therapy in patients with DDAF. The NOAH—AFNET 6 results were validated in a pre-specified secondary analysis of ARTESiA and meta-analyzed.”
About half of the study population of NOAH—AFNET 6 and ARTESiA (56% in NOAH—AFNET 6; 46% in ARTESiA) had concomitant vascular disease with an established indication for acetylsalicylic acid therapy. In these patients, stroke, myocardial infarction, systemic or pulmonary embolism, or cardiovascular death occurred less often with than without anticoagulation (3.9% versus 5.0% per patient-year in NOAH—AFNET 6 and 3.2% versus 4.4% per patient-year in ARTESiA).
Without vascular disease, outcomes were equal with and without anticoagulation (2.7% per patient-year in NOAH—AFNET 6 and 2.3% per patient-year in ARTESiA in both groups). Meta-analysis found consistent results across both trials.
Anticoagulation increased major bleeding in a comparable fashion in patients with vascular disease (edoxaban 2.1% per patient-year; no anticoagulation 1.3% per patient-year; apixaban 1.7% per patient-year; no anticoagulation 1.1% per patient-year) and without vascular disease (edoxaban 2.2% per patient-year; no anticoagulation 0.6% per patient-year; apixaban 1.4% per patient-year; no anticoagulation 1.1% per patient-year).
AFNET board chair Prof. Paulus Kirchhof, UKE, principal investigator of the NOAH—AFNET 6 trial, concluded, “This combined NOAH—AFNET 6 and ARTESiA sub-analysis suggests different effects of anticoagulation in DDAF patients with and without concomitant vascular disease. In the high-risk subgroup of patient with DDAF and vascular disease, anticoagulation therapy appears to reduce thromboembolic events with a greater magnitude than in patients without vascular disease. These data can guide shared clinical decision making on anticoagulation therapy in patients with DDAF.”
More information:
Paulus Kirchhof et al, Anticoagulation in device-detected atrial fibrillation with or without vascular disease: a combined analysis of the NOAH-AFNET 6 and ARTESiA trials, European Heart Journal (2024). DOI: 10.1093/eurheartj/ehae596
Provided by
Atrial Fibrillation NETwork
Citation:
Device-detected atrial fibrillation: Anticoagulation may have greater benefit in patients with vascular disease (2024, September 2)
retrieved 2 September 2024
from https://medicalxpress.com/news/2024-09-device-atrial-fibrillation-anticoagulation-greater.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.