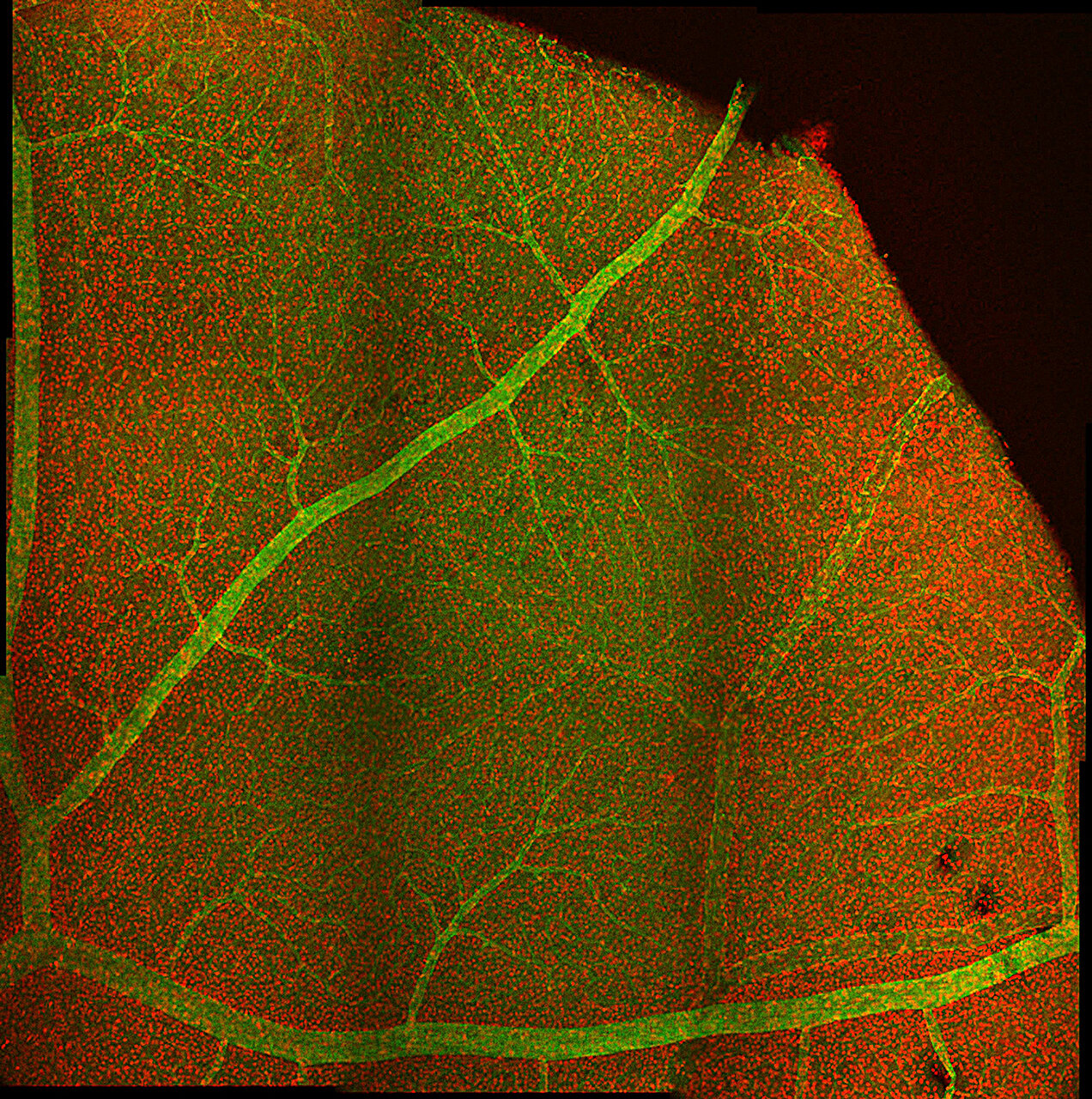
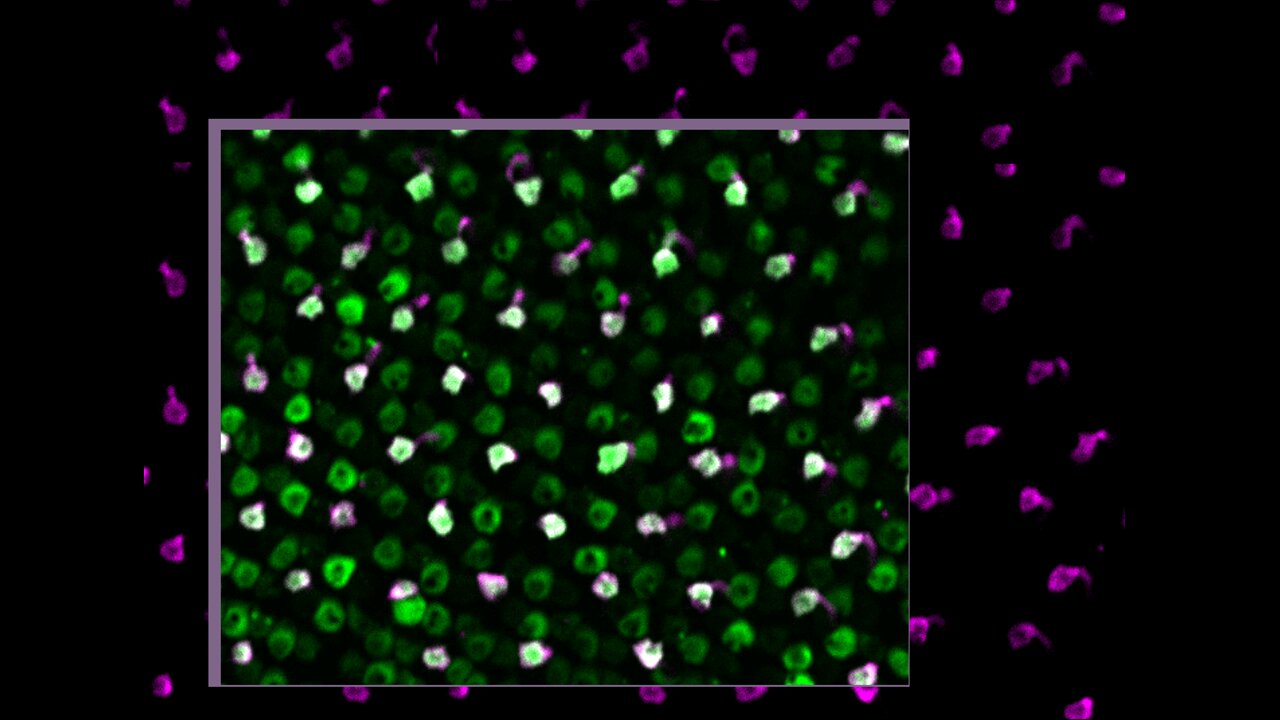
Researchers led by the University Hospital Zurich have identified cases of sterile intraocular inflammation, including severe retinal vasculitis, associated with faricimab injections used to treat eye conditions including age-related macular degeneration and diabetic macular edema.
Faricimab is a monoclonal antibody that targets vascular endothelial growth factors and angiopoietin pathways to stabilize blood vessels and reduce abnormal growth. Food and Drug Administration approved, it is commonly administered for neovascular age-related macular degeneration and diabetic macular edema. Clinical trials have previously demonstrated its efficacy and an acceptable safety profile.
In a retrospective case series study, “Sterile Intraocular Inflammation Associated With Faricimab,” published in JAMA Ophthalmology, researchers reviewed the records of seven patients referred to the ophthalmology department of the University Hospital Zurich between June 2022 and March 2024.
Twelve eyes of the seven patients developed inflammation after receiving faricimab intravitreal injections. Among these cases, two eyes experienced retinal vasculitis, with one progressing to irreversible vision loss due to macular capillary nonperfusion.
The median time between the last faricimab injection and the onset of inflammation was 28 days. Most cases involved moderate inflammation treated with corticosteroids without significant vision changes. The severe cases resulted in substantial and permanent vision impairment, raising concerns that rare adverse events may not have been captured in clinical trials that led to faricimab approval.
In one instance, an 83-year-old woman developed occlusive vasculitis in both arteries and veins of the eye, leading to a drop in visual acuity from 20/80 to 20/2000.
Retrospective case series studies cannot establish cause-and-effect relationships, only find connections between patients with a similar condition. Research involving larger patient populations with specific data collection would be needed to clarify the safety profile of faricimab in real-world settings.
In the meantime, the findings suggest more intensive follow-up is needed for patients receiving faricimab treatment. Vigilant monitoring allows for prompt identification and treatment of these cases, which might be enough to prevent long-term eye damage.
More information:
Mariano Cozzi et al, Sterile Intraocular Inflammation Associated With Faricimab, JAMA Ophthalmology (2024). DOI: 10.1001/jamaophthalmol.2024.3828
© 2024 Science X Network
Citation:
Adverse effects found following faricimab treatment for eye conditions (2024, October 14)
retrieved 16 October 2024
from https://medicalxpress.com/news/2024-10-adverse-effects-faricimab-treatment-eye.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.