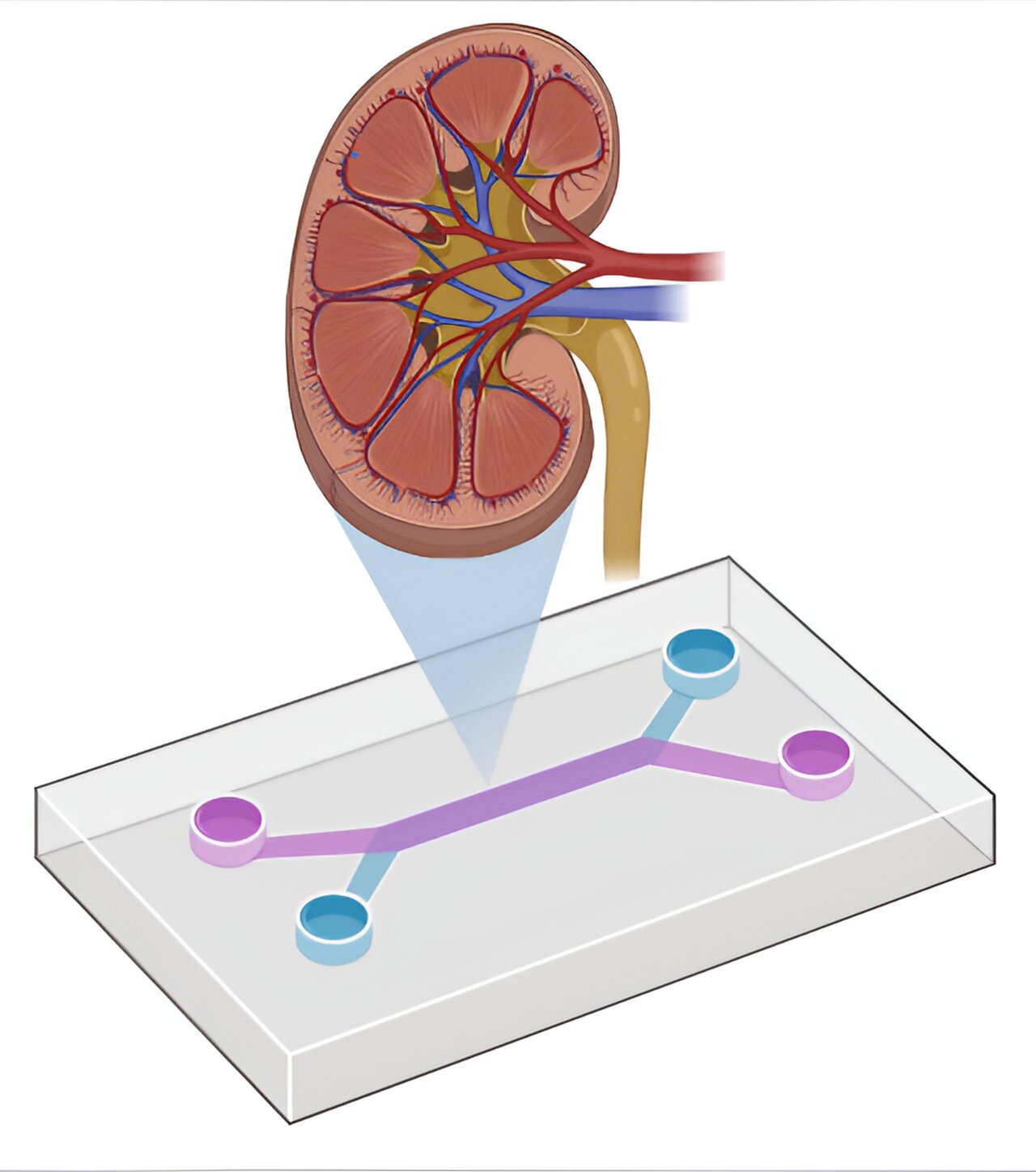
Developing advanced drug screening tools is crucial for the advancement of personalized medicine and the creation of more effective treatments. One organ receiving particular attention in this area is the kidney.
For example, the kidney’s proximal tubules are essential for reabsorbing critical substances from the bloodstream before urine formation. However, traditional in vitro models have struggled to accurately replicate this, often failing to express key transport proteins like organic anion transporters—OAT1/3—and organic cation transporter 2—OCT2.
A team at Kyoto University has now developed a human iPS cell-derived kidney organoid-based proximal tubule-on-chip—OPTECs-on-Chip—that mimics in vivo renal physiology more closely than ever before. This model exhibits enhanced expression and polarity of essential renal transporters, making it a powerful tool for assessing drug transport and nephrotoxicity. The paper is published in the journal iScience.
“Our OPTECs-on-Chip demonstrates significant improvements in the expression and functionality of OAT1/3 and OCT2 transporters compared to previous models using immortalized cells,” explains lead author Cheng Ma from KyotoU’s Graduate School of Engineering.
This microphysiological system—MPS—utilizes two widely adopted differentiation protocols to derive kidney organoids, integrating them into a microfluidic system to form a proximal tubule model. This successfully maintains transporter expression, replicating the mechanisms of drug excretion in renal proximal tubules in vitro, mimicking the function of human epithelial tissue.
“Listening to the needs of pharmaceutical companies to develop the high-function kidney chip they require is the best way for us to integrate MPS technology into drug development,” explains team leader Ryuji Yokokawa at KyotoU’s Department of Micro Engineering.
“We demonstrated that our OPTECs-on-Chip not only assesses nephrotoxicity but also quantifies transcellular substrates transported specifically by OAT1, OAT3, and OCT2. This highlights the benefits of using iPS cell-derived cells and a microfluidic system to replicate in vivo cellular transport mechanisms,” add co-author Minoru Takasato at the RIKEN Center for Biosystems Dynamics Research, together with Toshikazu Araoka of KyotoU’s Center for iPS Cell Research and Application.
Yokokawa’s team anticipates applying their MPS model as a screening tool for developing new drugs by evaluating the transport and nephrotoxicity of various membrane proteins.
“Our model has significant potential for drug screening and personalized medicine,” notes Yokokawa. “By incorporating patient-derived stem cells, we can develop personalized assessments for renal transport and disease modeling.”
More information:
Cheng Ma et al, Efficient proximal tubule-on-chip model from hiPSC-derived kidney organoids for functional analysis of renal transporters, iScience (2024). DOI: 10.1016/j.isci.2024.110760
Citation:
Advanced drug screening: A microphysiological system to assess drug transport and nephrotoxicity (2024, September 18)
retrieved 21 September 2024
from https://medicalxpress.com/news/2024-09-advanced-drug-screening-microphysiological-nephrotoxicity.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.