Exercise intolerance is often severe among patients with cardiovascular disease and can impose significant limitations on their physical abilities and quality of life. Medications known as cardiac myosin inhibitors (CMIs) are being developed to help patients with hypertrophic obstructive cardiomyopathy (HOCM), a disease in which the heart muscle becomes thickened, leading to reduced blood flow out of the heart. In a new analysis led by researchers from Mass General Brigham, investigators probed multiple exercise response patterns before and after exposure to the CMI aficamten in the SEQUIOA-HCM trial.
Results are published today in JAMA Cardiology.
“My laboratory has had a longstanding focus on understanding mechanisms of exercise intolerance and exploration of therapeutic interventions to improve exercise capacity,” said corresponding author Gregory Lewis, MD. the Section Head of Heart Failure and Director of Cardiopulmonary Exercise Testing at Massachusetts General Hospital, a founding member of the Mass General Brigham health care system.
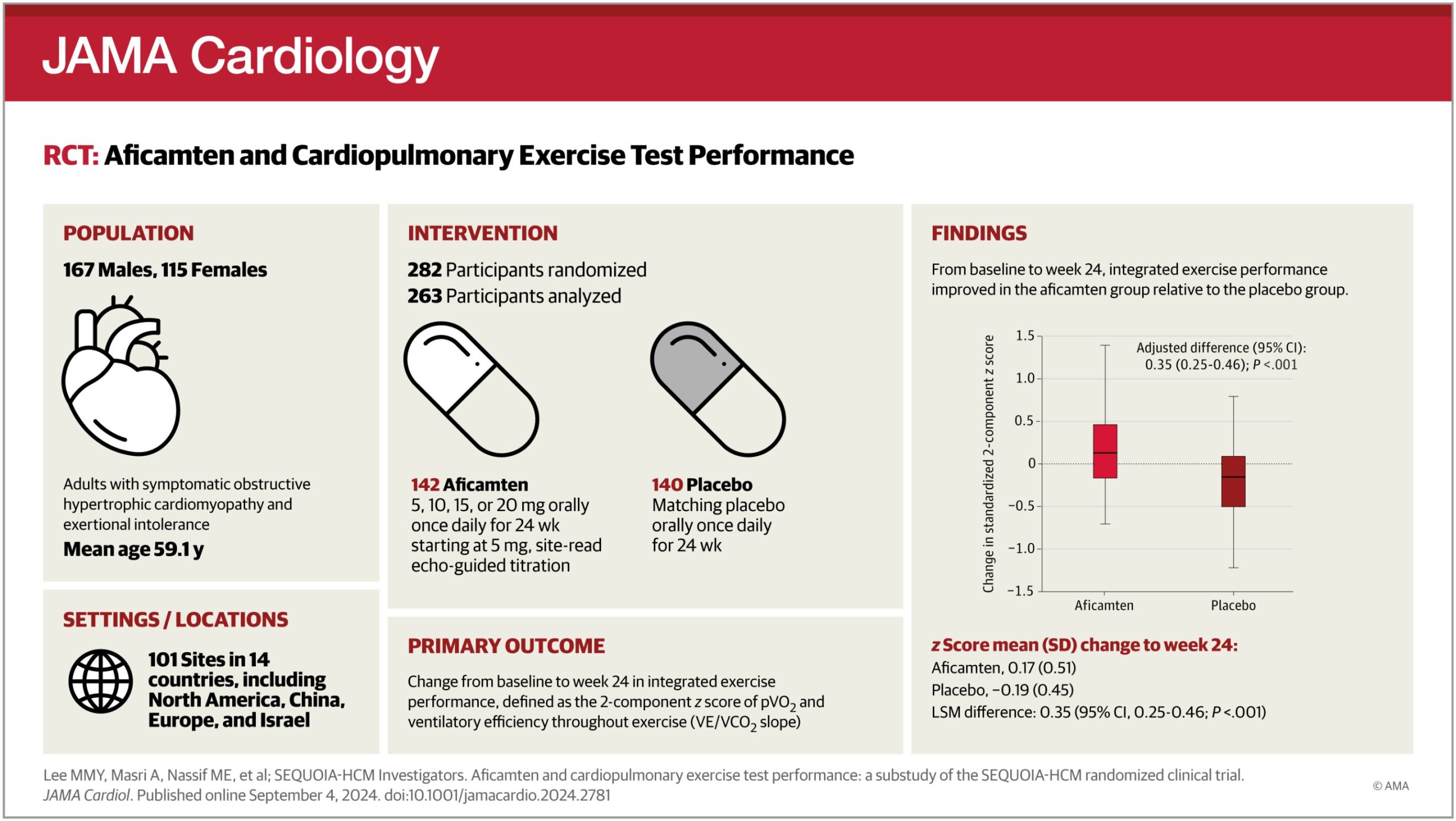
The phase-3, placebo-controlled, randomized clinical trial SEQUIOA-HCM evaluated effects of aficamten on exercise capacity and cardiac function. The primary endpoint of the study was change in peak oxygen uptake as measured by cardiopulmonary exercise testing under the oversight of the MGH Cardiopulmonary Exercise Testing Core Laboratory directed by Lewis.
The study found marked improvements in both peak oxygen uptake as recently reported in the New England Journal of Medicine. In addition, cardiac structure and function measures improved significantly, as adjudicated by the Echocardiography Core Laboratory directed by Scott Solomon, MD, from Brigham and Women’s Hospital, a founding member of the Mass General Brigham health care system.
The team assessed 263 patients who completed exercise testing at the start of the trial and 24 weeks later. They saw significant improvements in several measurements, including workload achieved, breathing efficiency and cardiac power generated during exercise, and submaximum exercise capacity measured at the anaerobic threshold.
In addition, they describe a novel measurement that combined submaximum and maximum exercise capacity as an integrated way to assess exercise capacity improvement. The investigators found that changes in exercise responses related to changes in cardiac structure and function beyond just outflow obstruction.
Their findings have important clinical implications for treating HOCM and could also be relevant to broader populations of non-obstructive hypertrophic cardiomyopathy.
More information:
Aficamten and Cardiopulmonary Exercise Test Performance A Substudy of the SEQUOIA-HCM Randomized Clinical Trial, JAMA Cardiology (2024). DOI: 10.1001/jamacardio.2024.2781
Citation:
Heart drug improves exercise tolerance in clinical trial of patients with hypertrophic obstructive cardiomyopathy (2024, September 4)
retrieved 4 September 2024
from https://medicalxpress.com/news/2024-09-heart-drug-tolerance-clinical-trial.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.