The ability to therapeutically manipulate the immune system presents a multitude of potential opportunities for treating infectious diseases, cancer and other diseases, but first, researchers must fully understand the processes that shape immunity.
Scientists at St. Jude Children’s Research Hospital have added another piece to the puzzle, showing how metabolic factors influence tissue immunity in an intricate interplay between nutrient availability, organelle biology and tissue-resident memory T (TRM) cell development.
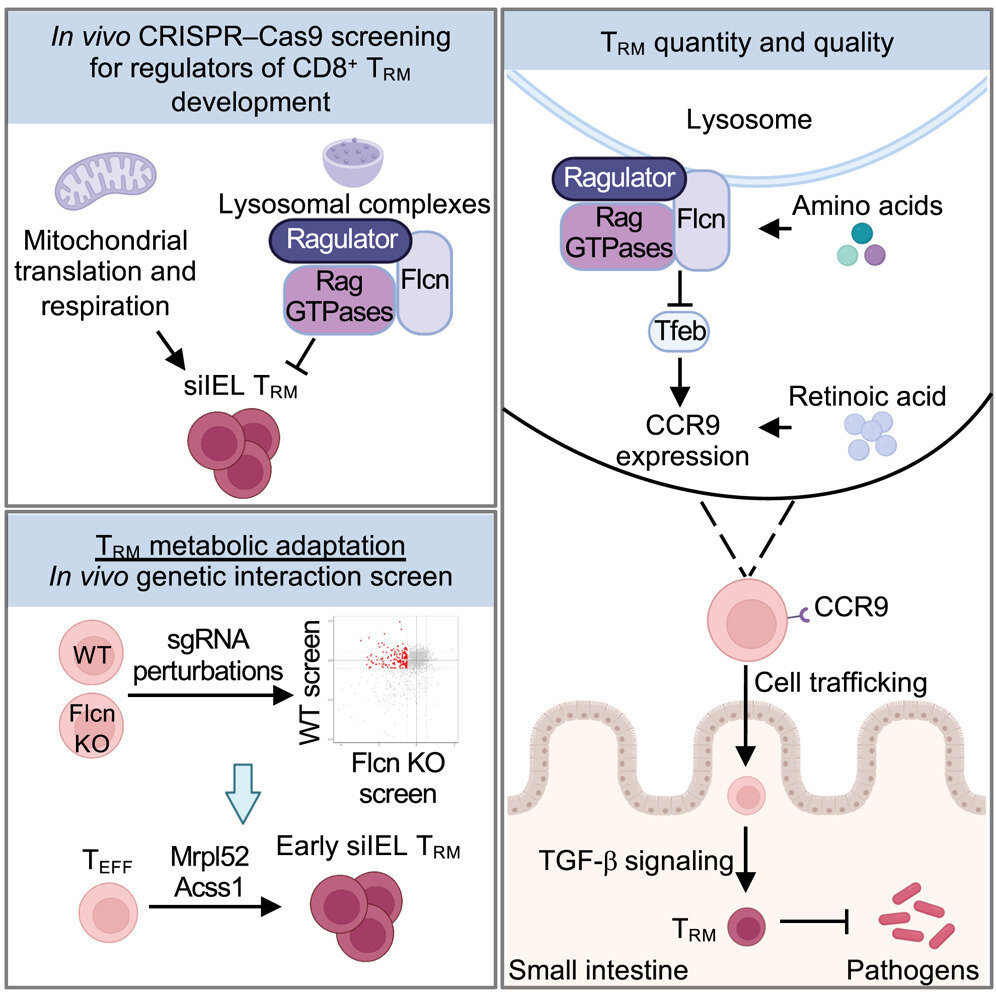
The team found that TRM cells rely on mitochondrial activity for development and are inhibited by nutrient-sensing pathways in lysosomes. The findings were published today in Immunity.
“We are excited to introduce a new layer of regulation in tissue immunity, which we believe will significantly enhance our understanding of this field. Our findings have the potential to inform new strategies for modulating tissue immunity, particularly for improving anti-infection responses,” said corresponding author Hongbo Chi, Ph.D., St. Jude Department of Immunology.
The crucial role of memory T cells
CD8+ T cells are vital in defending against infections and cancer while promoting tissue health. During primary infections, they differentiate into effector cells, which provide immediate responses, and memory cells, which persist long-term for quicker responses upon re-exposure.
TRM cells are a specialized subset of memory cells that remain in tissues, offering rapid, localized responses by monitoring for signs of infection. Their ability to act as first responders enhances tissue-specific immunity, making them crucial for effective adaptive or long-term immunity.
“Our study focuses on tissue immunity, specifically examining TRM cell formation,” said first author Jana Raynor, Ph.D., St. Jude Department of Immunology. “These cells migrate to specific tissues—such as the lungs, intestines or skin—and establish long-term residency.
“They help provide the first line of defense against infections, and TRM-like cells are increasingly recognized for their role in responding to tumors.”
TRM and TRM-like cells are of great interest due to their potential use in immunotherapy since they provide rapid, localized immune responses at the site of infection or tumor growth. Their long-term presence in tissues ensures lasting immunity and strong immune memory, allowing rapid reactions to previously encountered pathogens or cancer cells.
Organelle signaling and immune cell development
The researchers used CRISPR-Cas9 genetic screens to uncover critical signaling pathways influencing TRM cell development and differentiation. They found that TRM cell formation depends on processes in cellular organelles called mitochondria. Additionally, signaling nodes at the lysosome (another organelle), such as Folliculin (Flcn), Ragulator and Rag GTPases, restrict TRM formation and development.
“What we found particularly intriguing was that Flcn-mediated regulation is specific to the intestine, making this the first identified negative regulator of TRM cells at a localized site,” said Raynor. “We’re excited about targeting this pathway to enhance long-term TRM cells in the gut, as our study shows that increased Flcn-deficient TRM cell levels provide better protection against infections.”
Apart from organelle signaling, the study also found that nutrient availability played a role in tissue immunity. Specifically, Flcn modulates the activity of transcription factor EB (Tfeb). Flcn–Tfeb signaling, induced by amino acid deprivation, contributes to TRM cell development.
The relationship links nutrient stress to cell fate decisions. Therefore, the Flcn–Tfeb axis is a regulatory pathway that coordinates immune responses to nutrient availability and lysosomal function.
“T cells migrate to various tissues and must adapt to the local nutrient environment. Our research uncovers the mechanisms by which T cells adjust to extracellular nutrient conditions and connect them to a key intracellular organelle, lysosome,” said Chi.
“This discovery further cements the emerging concept we helped champion that nutrients serve as a unique type of signal, known as ‘Signal 4,’ to license T-cell immunity.”
This work underscores metabolism-associated mechanisms that drive immune memory response to occur in a highly tissue-specific manner, showcasing promising future applications such as dietary interventions, vaccination strategies and personalized medicine approaches.
“We’re particularly excited about the potential of nutrient availability and diet, as it’s something individuals can actively manage and apply. Exploring how to modulate nutrition may significantly influence the establishment of stronger immune responses,” added Raynor.
More information:
Jana L. Raynor et al, CRISPR screens unveil nutrient-dependent lysosomal and mitochondrial nodes impacting intestinal tissue-resident memory CD8+ T cell formation, Immunity (2024). DOI: 10.1016/j.immuni.2024.09.013
Citation:
Researchers show how nutrients and organelle signaling shape tissue immunity (2024, October 15)
retrieved 16 October 2024
from https://medicalxpress.com/news/2024-10-nutrients-organelle-tissue-immunity.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.