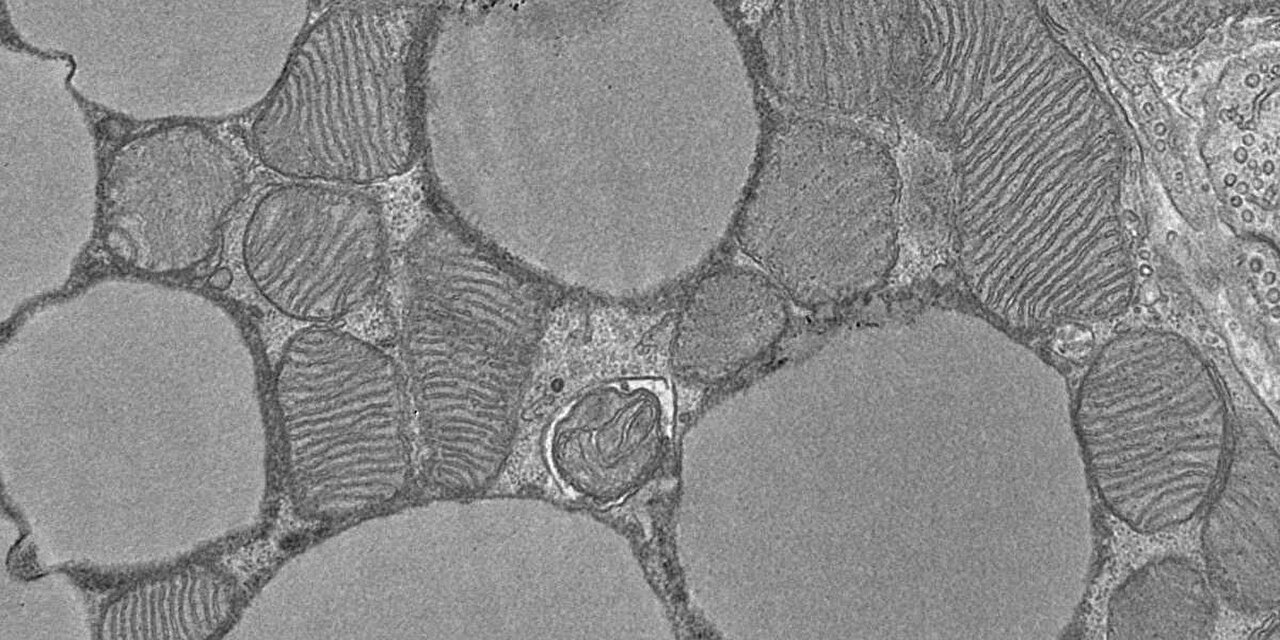
Special fat cells known as brown adipocytes help maintain body temperature by converting calorie-rich nutrients into heat. This protects us from gaining excess weight and from metabolic disorders.
An international team of researchers led by Professor Alexander Bartelt from the Institute for Cardiovascular Prevention (IPEK) has deciphered a new mechanism that increases respiration and metabolic activity of brown fat cells. The researchers hope that this discovery will lead to novel approaches utilizing brown fat against metabolic diseases.
Their results were recently published in The EMBO Journal.
The activation of fat-burning cells makes people lose weight. When it is cold, brown adipocytes extract their fuel from storage fat, as thermogenesis requires a lot of calories. “People who train their brown fat through regular cold exposure are thinner and less prone to developing diabetes and cardiovascular diseases,” says Bartelt.
Brown fat cells are particularly rich in mitochondria, the power plants where cellular respiration takes place. However, science does not yet sufficiently understand how brown fat cells boost metabolism such that new therapies could be developed.
Cold stimulates thermogenesis
The molecular trick of brown fat cells is uncoupling protein-1, which ensures that heat is generated instead of ATP, the conventional product of cellular respiration. “The high metabolic activity of brown fat cells must also influence the production of ATP,” says Bartelt, “and we hypothesized that this process would be regulated by cold.”

Together with Brazilian colleagues from São Paulo, the researchers identified “inhibitory factor 1,” which ensures that ATP production is maintained instead of thermogenesis. When the temperature goes down, the levels of inhibitory factor-1 fall and thermogenesis can take place. When artificially increased, inhibitory factor 1 disrupts the activation of brown fat in the cold.
These findings were obtained in isolated mitochondria, cultivated cells, and an animal model. “While we have found an important piece of the puzzle for understanding thermogenesis, therapeutic applications are still a long way off,” explains Dr. Henver Brunetta, who conducted the study.
According to the authors, most people use their brown fat too little and it becomes dormant. The new study results indicate that there are molecular switches that allow mitochondria of brown fat cells to work better.
Bartelt and his colleagues plan to build on this discovery. “Ideally, we’ll find new ways, based on our data, to also restore the fitness of mitochondria in white fat cells, as most people have plenty if not too many of them,” concludes Bartelt.
More information:
Henver S Brunetta et al, IF1 is a cold-regulated switch of ATP synthase hydrolytic activity to support thermogenesis in brown fat, The EMBO Journal (2024). DOI: 10.1038/s44318-024-00215-0
Citation:
Brown fat: How cells generate heat by burning calories (2024, September 16)
retrieved 6 October 2024
from https://medicalxpress.com/news/2024-09-brown-fat-cells-generate-calories.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.