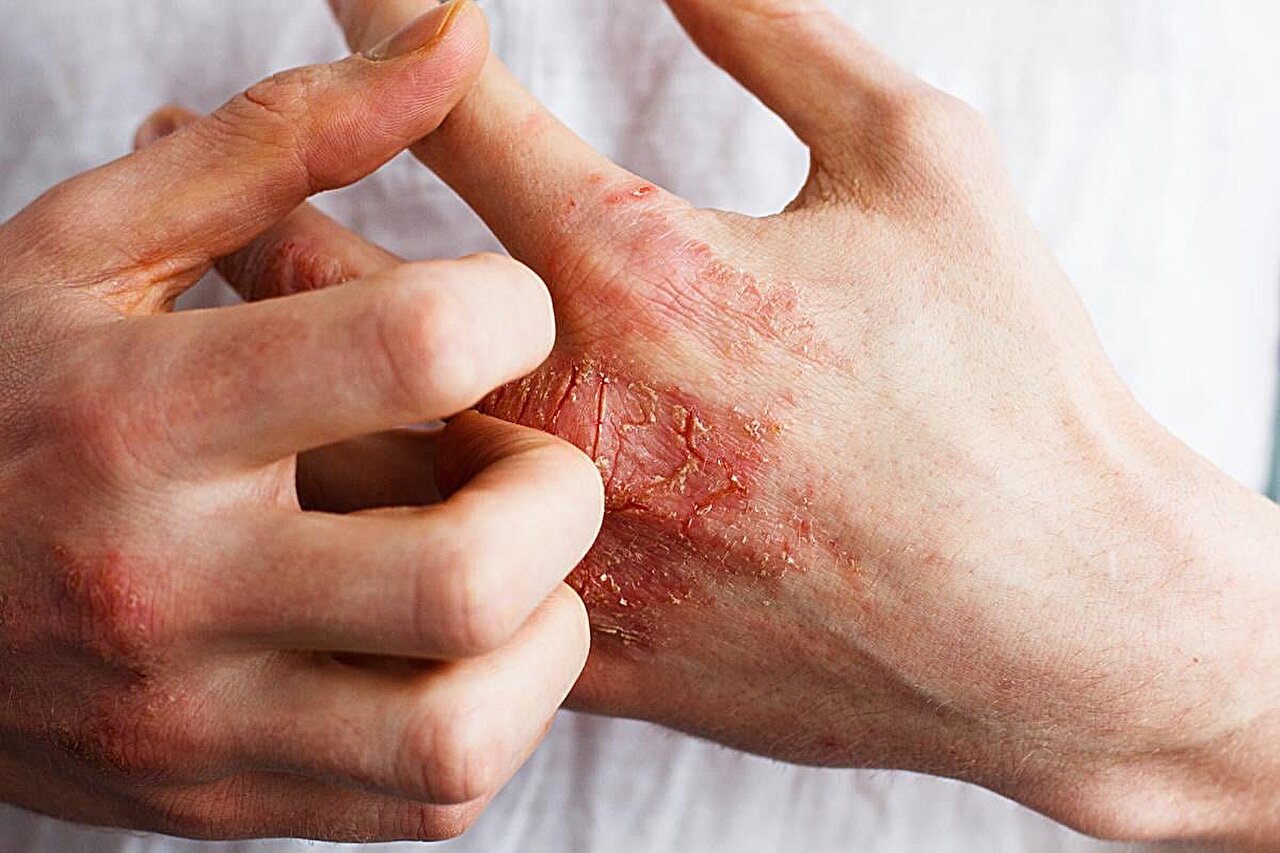
For patients with moderate-to-severe atopic dermatitis (AD), lebrikizumab has long-term efficacy, according to a study presented at the annual meeting of the European Academy of Dermatology and Venereology, held from Sept. 25 to 28 in Amsterdam.
Diamant Thaçi, Ph.D., from University Lübeck in Germany, and colleagues reported long-term efficacy and safety from the ADjoin long-term extension study. Adults aged 18 years and older and adolescents aged 12 to
Patients receiving lebrikizumab who met protocol-defined response criteria after 16 weeks were randomly assigned to receive LEBQ2W, lebrikizumab 250 mg every four weeks (LEBQ4W), or placebo. Participants who completed 52 weeks were eligible to enroll in ADjoin and received the same treatment regimen. Efficacy was assessed though week 100 of ADjoin.
Overall, 82 patients receiving LEBQ2W and 99 receiving LEBQ4W entered ADjoin. The researchers found that of the patients with Investigator Global Assessment (IGA) scores of 0/1 at week 16 in the LEBQ2W and LEBQ4W groups, respectively, 81.5 and 83.3% maintained IGA 0/1 at week 52 (ADjoin week 0) and 82.9 and 84.0% maintained IGA 0/1 at week 152.
Of the patients with Eczema Area and Severity Index 75% improvement (EASI 75) at week 16 in the LEBQ2W and LEBQ4W groups, 96.3 and 93.7% and 90.5 and 94.1% maintained EASI 75 at week 52 and week 152, respectively. Throughout ADjoin, 14.6 and 24.2% of those receiving LEBQ2W and LEBQ4W, respectively, used any rescue therapy.
“These three-year results provide compelling evidence of durable efficacy and a consistent safety profile,” senior author Eric Simpson, M.D., from Oregon Health & Science University in Portland, said in a statement.
The study was funded by Eli Lilly, the manufacturer of lebrikizumab.
© 2024 HealthDay. All rights reserved.
Citation:
Long-term efficacy for lebrikizumab seen in moderate, severe eczema (2024, October 5)
retrieved 5 October 2024
from https://medicalxpress.com/news/2024-10-term-efficacy-lebrikizumab-moderate-severe.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.